Ring closure of hydroxythiourea
SyntheticPage 603
DOI:
Submitted: July 8, 2013, published: July 10, 2013
Authors
Edward J Crust (e.j.crust@warwick.ac.uk)
Pratik Gurnani (pratik.gurnani@warwick.ac.uk)
A contribution from
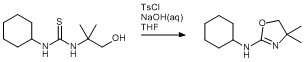
Chemicals
NaOH
p-toluenesulfonyl chloride (Alfa Aesar)
THF (distilled over potassium)
Procedure
A Schlenk vessel containing the cyclohexyl thiourea (2.0 g, 8.68 mmol) was dissolved in THF (20 mL) and purged with argon. An emulsion of NaOH (0.87 g, 0.2 mol) in water (~3 mL) and p-toluenesulfonyl chloride (1.83 g, 9.60 mmol) in THF (~7 mL) was added slowly (~20 min). The solution was then left to stir overnight. The product was then extracted with dichloromethane and dried over magnesium sulphate. The solvent was then removed by evaporation under reduced pressure to yield a white solid (yield 1.22 g, 72 %).
Author Comments
Data
1H NMR (293 K, d1-chloroform) d 1.11 (m, CH2, 2H), 1.23 (s, 6H, CH3), 1.35 (m, 2H, CH2), 1.54 (m, 2H, CH2), 1.67 (m, 2H, CH2), 1.98 (m, 2H, CH2), 3.37 (m, 1H, CH), 3.64 (br s, 1H, NH).
13C{1H} NMR (293 K d1-chloroform) d 24.7 (cyclohexyl-CH2), 25.5 (cyclohexyl-CH2), 28.2 (cyclohexyl-CH2), 28.8 (CH3), 33.5 (cyclohexyl-CH2), 51.2 (cyclohexyl-CH), 65.0 (Me2Cq), 78.8 (CH2), 158.3 (NCqN).
IR (Thin film, cm-1): 3192, 2928, 2854, 1655, 1609, 1553, 1451, 1350, 1319, 1293, 1247, 1202, 1165, 1119, 1027, 974, 891, 703, 673.
Anal. Calcd. for C11H20N2O: C, 67.31; H, 10.27; N, 14.27. Found: C, 67.22; H, 10.24; N, 14.21.
MS (EI) m/z 196 (M+)
Lead Reference
E. J. Crust, I. J. Munslow and P. Scott, Journal of Organometallic Chemistry, 2005, 690, 3373-3382.http://dx.doi.org/10.1016/j.jorganchem.2005.04.019
Keywords
amines, aminooxazoline, intramolecular, thiourea