Buchwald-Hartwig amination of a bromopyridine with cyclohexane-1,2-diamine
SyntheticPage 602
DOI:
Submitted: July 8, 2013, published: July 10, 2013
Authors
Edward J Crust (e.j.crust@warwick.ac.uk)
Pratik Gurnani (pratik.gurnani@warwick.ac.uk)
A contribution from
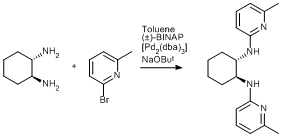
Chemicals
2-bromo-6-methyl pyridine (Aldrich)
(±)-BINAP
[Pd2(dba)3] (Aldrich)
NaOBut (Aldrich)
Toluene (distilled over sodium)
Procedure
Toluene (50 mL) was added to a large Schlenk vessel charged with (+/-)-trans‑1,2‑diaminocyclohexane (1.0 g, 8.8 mmol), 2-bromo-6-methyl pyridine (3.0 g, 18 mmol), (±)-BINAP (218 mg, 0.35 mmol), [Pd2(dba)3] (160 mg, 0.18 mmol) and NaOBut (2.4 g, 25 mmol). The resulting deep red/brown mixture was heated for 4 h at 80°C with stirring. On cooling to room temperature diethyl ether (50 mL) was added. The resultant yellow mixture was washed with brine (2 x 30 mL), dried over MgSO4 and the solvent removed by evaporation under reduced pressure. The yellow product was recrystallised from pentane / diethyl ether (yield 1.72 g, 60 %).
Author Comments
Data
1H NMR (293 K, d6-benzene) d 1.25 (m, 4H, cyclohexane-CH2), 1.58 (m, 2H, cyclohexane-CH2), 2.30 (m, 2H, cyclohexane-CH2), 2.52 (s, 6H, Py-CH3), 4.05 (m, 2H, ipso cyclohexane‑CH), 5.39 (s, 2H, NH), 5.97 (d, 3JHH = 7 Hz, 2H, Py-CH), 6.34 (d, 3JHH = 7 Hz, 2H, Py-CH), 7.06 (t, 3JHH = 7 Hz, 2H, Py-CH).
13C{1H} NMR (293 K d6-benzene) d 25.1 (Py-CH3), 25.6 (cyclohexane-CH2), 33.6 (cyclohexane-CH2), 56.5 (cyclohexane-CH), 105.9 (Py-CH), 111.6 (Py-CH), 137.6 (Py-CH), 157.0 (PyCqCH3), 159.3 (PyNCqN).
IR (nujol, cm-1): 3292, 2726, 2669, 1605, 1582, 1519, 1336, 1295, 1226, 1153, 1115, 1099, 1029, 986, 948, 933, 778, 728.
Anal. Calcd. for C18H24N4: C, 72.94; H, 8.16; N, 18.90. Found: C, 73.06; H, 8.09; N, 18.86.
MS (CI) m/z 297 (M+), 202 (M+-PyCH3), 187 (M+-NH and PyCH3), 109 (M+-2 x PyCH3).
Lead Reference
E. J. Crust, I. J. Munslow and P. Scott, Journal of Organometallic Chemistry, 2005, 690, 3373-3382.http://dx.doi.org/10.1016/j.jorganchem.2005.04.019
Keywords
2-diaminocyclohexane, addition, amination, amines, aromatics/arenes, Buchwald-Hartwig, trans-1 2-bromo-6-methyl pyridine, transition metal catalysed