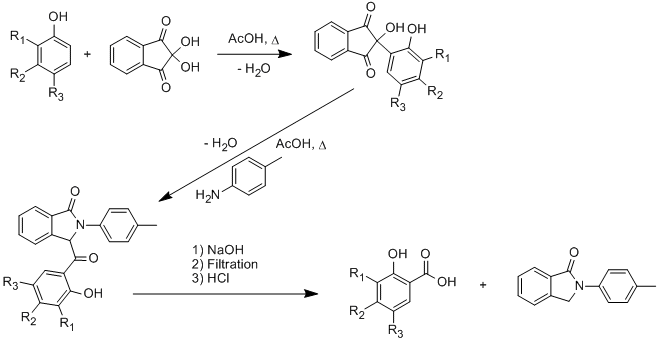
Condensation of phenols with ninhydrin
SyntheticPage 6
DOI:
Submitted: June 11, 2001, published: June 11, 2001
Authors
Ian Munslow (msrmz@csv.warwick.ac.uk)
Chemicals
phenol (1 equiv.)
ninhydrin (1 equiv.)
4-methylaniline (2 equiv.)
acetic acid (100 ml / 0.1 mol)
2N sodium hydroxide (30 ml / 0.05 mol)
H2O (10 ml / 0.05 mol)
6N hydrochloric acid (10 ml / 0.05 mol)
ninhydrin (1 equiv.)
4-methylaniline (2 equiv.)
acetic acid (100 ml / 0.1 mol)
2N sodium hydroxide (30 ml / 0.05 mol)
H2O (10 ml / 0.05 mol)
6N hydrochloric acid (10 ml / 0.05 mol)
Procedure
The phenol is added to a hot solution of ninhydrin in acetic acid and the mixture heated at reflux for 2h. After cooling to room temperature the resulting precipitate was isolated by vacuum filtration (1), washed with ice-cold acetic acid and dried in vacuo, 2nd crop can be collected. 4-Methylaniline is added to a solution of (1) in acetic acid and stirred under reflux for 12h The mixture is allowed to cool to room temperature before the resulting precipitate was isolated by vacuum filtration, washed with ice-cold acetic acid and dried in vacuo, 2nd crop can be collected. (2) (2) is added to aqueous 2N NaOH and refluxed for 15 min, resulting white ppt was isolated by filtratrion and washed with water. Yellow filtrate and washing were then cooled and acidified with 6N HCl, resulting white ptp is isolated by vacuum filtration, washed with cold water and recrystallized from ethanol/water to give a white solid.
Author Comments
Procedure works well on the large scale, several grams of pure salicyclic acid being available in a day or two. Each step has crystalline end products, no purification is needed. Compounds made: 3-iso-propyl-2-hydroxybenzoic acid, 3,5-di-tert-butyl-2-hydroxybenzoic acid, 3,5-di-methyl-2-hydroxybenzoic acid.
Data
Depends on the starting phenol. 3-iso-Propyl-2-hydroxybenzoic acid 1H NMR (CDCl3): 10.91 (brs, 1H, OH), 7.82 (d, J = 6, 1H, Ar-H), 7.45 (d, J = 6, 1H, Ar-H), 6.88 (t, J = 6, m1H, Ar-H), 4.71 (brs, 1H, OH), 3.38 (sept, J = 7, 1H, CHMe2), 1.23 (d, J = 7, 6H, CHMe2).
Lead Reference
Schmitt, G. Synthesis 1984, 758.
Keywords
Comments
I have repeated this procedure several times, using 2-tBu, 4-OMe phenol, and the product after the second reaction does not precipitate from the reaction mixture on cooling. Pouring the reaction mixture onto water (~500 ml) with overhead stirring forms an impure ppt of product with some of the excess 4-methylaniline trapped in the matrix. Washing with cold diethyl ether removes the aniline, and the rest of the prep proceeds as above.
By Max Hammond on March 4, 2005