Allyl Grignard addition to the internal alkyne carbon of propargyl alcohol
SyntheticPage 596
DOI:
Submitted: June 2, 2013, published: June 7, 2013
Authors
John MacMillan (john.macmillan@temple.edu)
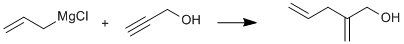
Chemicals
Propargyl alcohol, 99%, Sigma Aldrich, redistilled
Ether, anhydrous, 99+%, Sigma Aldrich, freshly opened cans
Tetrahydrofuran, 99+%, Sigma Aldrich, freshly opened cans
Magnesium, chip, 99.98%, Sigma Aldrich
Iodine, 99.98%,Sigma Aldrich
Magnesium sulfate,anhydrous, Sigma Aldrich
Ammonium chloride, 99.5%, Sigma Aldrich
Procedure
This compound, previously unreported, was prepared by the room temperature addition of propargyl alcohol to allyl magnesium chloride.
To a 5 liter three neck flask, equipped with mechanical stirrer, Friedrich water condenser, Y tube, 1 liter pressure equalized dropping funnel, nitrogen inlet, and calcium chloride drying tube, were placed 72 g (3 moles) of magnesium turnings, 500 ml of dry ether and several crystals of iodine. Then 50 ml of a solution of 115 g (1.5 moles) redistilled allyl chloride in 500 ml of dry ether was added in one portion and the mixture stirred vigorously. After five minutes, the reaction commenced as evidenced by formation of a white solution and vigorous ether reflux. The remainder of the solution was then added drop wise over six hours. Midway through the addition, 50 ml of dry tetrahydrofuran was added to redissolve the white precipitated Grignard complex.
After completion of the chloride addition, the solution was stirred for one hour, then a solution of 28 g ( 0.5 mole) redistilled propargyl alcohol in 200 ml ether was added, dropwise, over a period of three hours. A vigorous reaction occurred resulting in considerable ether evaporation. At the end of the addition, 300 ml additional ether was added and the solution was then stirred for fifteen hours. The mixture was decomposed by pouring over a slurry of 100 g ammonium chloride in ~ 500 g ice with manual stirring in a two liter beaker, and the two clear layers were separated. The aqueous layer was extracted with 2x 200 ml ether portions.The combined ether layers were washed twice with 100 ml portions of water and dried over 10 g anhydrous magnesium sullfate for one hour in a 1 liter Erlenmeyer flask. The ether solution was decanted into a 1 liter beaker and the ether was removed, on a steam bath in a fume hood, yielding a light yellow liquid. Fractionation was accomplished in a 50 ml conical vial immersed in an oil heating bath maitained at ~ 100° , with a short distilling column and water aspirator pressure. Two fractions were analyzed by gas chromatography (5 ft x 0.25 inch Triton-X 305 column). The first fraction, b.p.75-80°, (30mm) weighed 16g, and contained 13.9 g 2-methylene-4-pentene-1-ol and an unidentified high boiling biproduct. The second fraction, b.p. 80-90 °, weighed 6.54 g and contained 3.18 g 2-methylene-4-penten-1-ol and 3.12 g biproduct. The fractions were combined and the components separated by preparative gas chromatography utilizing a 8 ft x 1 inch column packed with Triton X 305, yielding 17g (34.7% based on amount of propargyl alcohol), of pure 2-methylene-4-penten-1-ol. Other runs were typically in the 28-38% yield range.
Author Comments
Data
b.p.158-9ο nD27 1.4535,
Infrared spectrum (neat) showed bands at 3400(s), 3050(m), 2970(w), 2900(m), 1830(w, overtone), 1650(m), 1440(m), 1420(m), 1230(m), 1100(w), 1060 (m), 1030(m), 1000(m), 920(s), and 905 (m) cm-l.NMR: (δ scale), 60MHz, deuterochloroform solvent ,showed a singlet at δ1.92 (1H, hydroxyl, collapses on addition of D2O), a singlet at δ4.10 (2H,aliphatics), a doublet at δ2.82 (2H, allylic), a muitiplet centered at δ5.1 (4H, terminal vinyl), and a muitiplet centered at δ5.8 (1H, internal olefinic).
Lead Reference
Other References
Keywords
2-methylen-4-penten-1-ol, addition, alcohols, alkenes, alkynes, internal alkyne addition, organometallics