Acetalization under aprotic conditions
SyntheticPage 573
DOI:
Submitted: November 14, 2012, published: November 15, 2012
Authors
Jason Woolford (drjaywoolford@gmail.com)
A contribution from
Chemicals
ortho-Anisaldehyde, 99% (Sigma-Aldrich)
Allyloxytrimethylsilane, 98% (Sigma-Aldrich)
Trimethylsilyl trifluromethanesulfonate, >98% (Sigma-Aldrich)
Pyridine, distilled from calcium hydride and stored over KOH.
CH2Cl2, distilled from calcium hydride
MgSO4, anhydrous (Fisher scientific)
Et2O, (Fisher Scientific)
EtOAc, (Fisher Scientific)
Petroleum Ether, fraction boiling between 40-60°C, (Fisher Scientific)
Allyloxytrimethylsilane, 98% (Sigma-Aldrich)
Trimethylsilyl trifluromethanesulfonate, >98% (Sigma-Aldrich)
Pyridine, distilled from calcium hydride and stored over KOH.
CH2Cl2, distilled from calcium hydride
MgSO4, anhydrous (Fisher scientific)
Et2O, (Fisher Scientific)
EtOAc, (Fisher Scientific)
Petroleum Ether, fraction boiling between 40-60°C, (Fisher Scientific)
Procedure
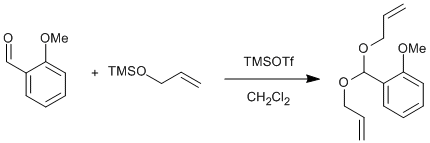
Trimethylsilyl trifluoromethanesulfonate (0.170 ml, 0.880 mmol), was added to CH2Cl2 (30 ml) under a nitrogen atmosphere at -84 °C (using a liquid N2/EtOAc bath), and stirred for five mins. Allyloxytrimethylsilane (36.4 ml, 220 mmol) and o-anisaldehyde (12.0 g, 88.0 mmol), dissolved in CH2Cl2 (25 ml) were added sequentially and drop wise, whilst maintaining the same temperature. The resulting yellow mixture was stirred at -84 °C for three hours, then allowed to warm to -60 °C (requires a chloroform/dry ice bath) and stirred for a further hour, the solution turning a deep red. The reaction was quenched with pyridine (15 ml) at the same temperature, and then poured into a saturated solution of NaHCO3 (100 ml). The aqueous layer was extracted with Et2O (3 x 100 ml); and the combined organic layers dried over anhydrous MgSO4. Excess solvent was removed under reduced pressure and the resulting oil was purified, when required, by flash column chromatography (EtOAc/petrol 2:98); to yield the acetal (20.2 g, 98%) as a colourless oil.
Author Comments
- Procedure adapted from that of Noyori, Tsunoda and Suzuki (see below)
- Useful for a range of different aldehydes, both aromatic and non-aromatic.
- Requires careful monitoring of temperature
- Easy to scale up.
- The triflate catalyst must be of good quality, preferrably newly purchased.
Data
1H NMR (500 MHz, CDCl3): δ 7.32 (1H, d, J 7.8); 7.24 (1H, t, J 8.0); 6.97 (1H, t, J 7.5); 6.91 (1H, d, J 8.0); 5.75-5.85 (2H, m); 4.96 (2H, d, J 17.2); 4.90 (2H, d, J 10.3); 3.87 (3H, s, -OCH3); 3.68 (1H, br. s, -OH); 2.10-2.18 (2H, ddm, J 2.7, 11.0); 1.99-2.09 (2H, m); 1.92 (4H, m)
13C NMR (126 MHz, CDCl3): δ 156.87, 139.24, 132.73, 128.02, 127.77, 120.80, 114.11, 111.51, 77.55, 55.43.
13C NMR (126 MHz, CDCl3): δ 156.87, 139.24, 132.73, 128.02, 127.77, 120.80, 114.11, 111.51, 77.55, 55.43.
Lead Reference
C.S. Penkett, J. A. Woolford, I. J. Day, M. P. Coles, J. Am. Chem. Soc., 2010, 123, 4. DOI: 10.1021/ja906163s
Other References
C. S. Penkett, J. A. Woolford, T. W. Read, R. J. Kahan. J. Org. Chem. 2011, 76, 1295 DOI: 10.1021/jo101927a
T. Tsunoda, M. Suzuki, R. Noyori, Tetrahedron Lett. 1980, 21, 1357. DOI: 10.1016/S0040-4039(00)74575-8
T. Tsunoda, M. Suzuki, R. Noyori, Tetrahedron Lett. 1980, 21, 1357. DOI: 10.1016/S0040-4039(00)74575-8
Supplementary Information
Keywords
Acetal formation, addition, aldehydes, aromatics/arenes, nucleophilic