Esterification of a carboxylic acid
SyntheticPage 567
DOI:
Submitted: July 25, 2012, published: April 3, 2013
Authors
Suzanne Elizabeth Howson (s.e.howson@warwick.ac.uk)
A contribution from
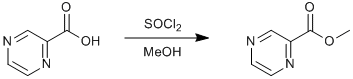
Chemicals
2-Pyrazinecarboxaldehyde (Sigma-Aldrich)
Thionyl chloride (Sigma-Aldrich)
Dry methanol - dried by heating to reflux for 3 d under dinitrogen over calcium hydride and degassed before use. Stored in glass ampoules under argon.
Sodium hydrogen carbonate (Fisher Scientific)
DCM (Fisher Scientific)
Sodium sulfate (Fisher Scientific)
Procedure
Author Comments
In the lead reference the crude product was recrystallised but I found the crude product to be of very high purity. Omitting this step led to a yield of 86% rather than 66% reported after recrystallisation.
Data
1H NMR (400 MHz, 298 K, CDCl3) δH 9.31 (1H, d, 4JHH = 1.5 Hz, Py), 8.76 (1H, d, 3JHH = 2.5 Hz, Py), 8.71 (1H, m, Py), 4.03 (3H, s, CH3).
13C{1H} NMR (100 MHz, 298 K, CDCl3) δC 164.5 (C=O), 147.9 (Py), 146.4 (Py), 144.5 (Py), 143.4 (Py), 53.3 (CH3).
Lead Reference
Keywords
aromatics/arenes, carboxylic acids, esterification, esters, nucleophilic, substitution