Boc Protection of Aminoglycosides
SyntheticPage 564
DOI:
Submitted: July 13, 2012, published: July 15, 2012
Authors
Brandon Findlay (brandon.findlay@zoho.com)
Chemicals
Boc anhydride
Triethylamine
Water
Methanol
Procedure
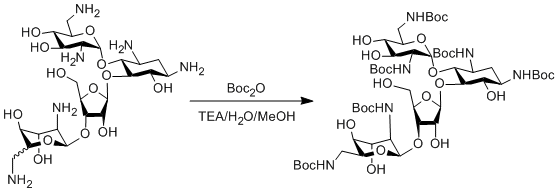
The amine of interest is dissolved in a 10/10/7 solution of water, methanol and triethylamine. As the solution is stirred Boc2O(s) (1.6eq) is slowly added, with a small amount of methanol used to rinse the weigh boat/paper. The solution is then heated to 55°C for 16hr (overnight), during which time the production of t-butanol will result in a thick slurry.
Following the reaction the solution is allowed to cool to room temperature and the stir bar is removed (if possible). The slurry is evaporated to dryness on a combined high vacuum/rotary evaporator setup, using dry ice as a coolant. The resulting white solid is then left under high vacuum for a minimum of 72hr, during which time residual Boc2O will sublimate into the dry ice trap. The product may then be used without further purification, extracted if the aminoglycoside was provided as a salt, or columned to separate out isomers (ie. neomycin B from neomycin C).
Author Comments
In this reaction, the starting material is a 85:15 mixture of neomycin B and neomycin C, which seems to be a fairly constant ratio among chemical suppliers. At the end of the reaction it is possible to isolate Boc-protected neomycin B via column chromatography (3:2 DCM:acetone). Yields for NBoc-neomycin B were ~60%, due to poor separation between the closely moving spots, but yields with tobramycin approached 97%.
Data
1H NMR (300 MHz, MeOD) δ 5.38 – 5.12 (m, 2H), 4.92 (s, 1H), 4.21 (s, 2H), 4.10 – 3.06 (m, 20H), 2.05 – 1.88 (m, 1H), 1.71 – 1.19 (m, 55H).
Lead Reference
Supplementary Information
Keywords
amines, carbamates, carbocyclic compounds, carbohydrates and sugars, heterocyclic compounds, protection