Grignard addition to aldehyde via chlorobenzene metalation
SyntheticPage 559
DOI:
Submitted: May 11, 2012, published: May 14, 2012
Authors
Ramesha Ramakrishna (ramesha63@hotmail.com)
A contribution from
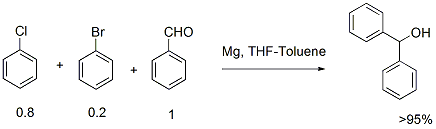
Chemicals
Bromobenzene-commercial grade
Toluene -commercial grade
Tetrahydrofuran commercial grade
Benzaldehyde from D. D. Fine Chem, LR grade
Magnesium turnings commercial grade
Ammonium chloride commercial grade
Procedure
Author Comments
1. It is always difficult to initiate Grignard reaction with chloro substrates. Many a times Grignard reaction stops after 50% reaction if the chloro substrates is used. One can overcome this problem by using a mixture of chloro (80-90%) and bromo substrate (20-10%).
2. Normally bromobenzene is 3-4 times expensive than chlorobenzene. Because of higher molecular weight of bromo compounds one has to use more quantity for the reaction. Therefore using chloro substrate is economically more viable.
3. Magnesium can be dried at 70-80 ofor 2-3 h for better initiation.
4. THF can be dried with KOH, just before the reaction. About 20-25 g of KOH is added to 100 mL THF and stirred for 3-4 min. The THF thus obtained is decanted to the reaction flask.
5. Toluene can be dried by distilling 10-15% and cooling the remaining toluene under nitrogen. This would remove any water present as azeotrope.
6. This is a practical method which can be used for any ketones/carbonyl compound.
Data
1H NMR (300 MHz): 7.50-7.20 (m, 10H), 5.83 (s, 1H), 2.20 (s,OH)
13C NMR (75 MHz): 142.00, 128.25, 127.30, 127.12, 79.92
Lead Reference
Other References
Keywords
addition, alcohols, aldehydes, alkyl/alkenyl/aryl halides, aromatics/arenes, chlorobenzene, Grignard, magnesium, nucleophilic