Finkelstein Reaction of 3-Chloro-1-Propanol with Sodium Iodide
SyntheticPage 551
DOI:
Submitted: March 30, 2012, published: April 6, 2012
Authors
Christopher Kelly (christopher.b.kelly@uconn.edu)
A contribution from
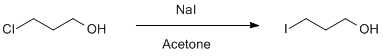
Chemicals
3-Chloro-1-Propanol (98% Purity, Sigma Aldrich)
Acetone (CHROMASOLV®, for HPLC, ≥99.9%, Sigma Aldrich)
Sodium Iodide (ACS reagent, ≥99.5%, Sigma Aldrich)
Diethyl Ether (anhydrous, ≥99.7%, 1 ppm BHT, Sigma Aldrich)
Hexanes (mixture of isomers, anhydrous, ≥99%, Purchased from Sigma Aldrich)
Sodium thiosulfate (ReagentPlus®, 99%)
Sodium Sulfate (ACS reagent, ≥99.0%, anhydrous, granular)
Procedure
To a 100 mL flask equipped a large stir bar was added the 3-chloro-1-propanol (4.73 g, 0.050 mol) and acetone (50 mL, 1 M in the alcohol). Sodium iodide (37.47 g, 0.250 mol) was then added all at once to the flask . The flask was then equipped with a reflux condenser and, after placing the reaction under a nitrogen atmosphere, was heated to reflux. The mixture was allowed to react for 24 hours. After this time, the now yellow solution was filtered through a medium porosity fritted funnel, eluting with ≈200 mL of acetone into a 500 mL round-bottom flask.1 The solvent was removed by rotary evaporation to afford a dry orange solid. This solid was triturated with a 1:1 v./v. hexanes to Et2O solution (2 X 200mL) and the grey fine precipitate was removed by filtration through a medium porosity fritted funnel.2 The slightly clouded3 yellow-tinged filtrate was washed with ≈100 mL of a 10% w./w. sodium thiosulfate solution, causing the organic layer to become colorless. The organic layer was then washed with ≈100 mL of deionized water, followed by ≈100 mL of brine, and dried with sodium sulfate. The solvent was removed by rotary evaporation to afford pure 3-iodo-1-propanol as a pale yellow oil (7.78 g, 84%).
Author Comments
1, Note that during this time the excess sodium iodide began to precipitate out as a bright orange solid. By rinsing with excess acetone, any trapped product is released into the flask, improving yield.
2. A spatula was used to break up any large orange aggregates
3. This cloudiness if likely some residual sodium iodide which is removed during washing
Data
13C NMR (100 MHz, CDCl3) δ ppm 3.25 (-CH2-I) 35.84 (-CH2-) 62.59 (-CH2-OH)
Lead Reference
Keywords
alcohols, Alkanes, Alkyl Iodides, Halogens, nucleophilic, substitution