Bromination of Diethylene Glycol
SyntheticPage 545
DOI:
Submitted: March 21, 2012, published: March 27, 2012
Authors
Rebecca Kaner (r.a.kaner@warwick.ac.uk)
A contribution from
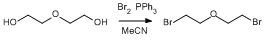
Chemicals
Bromine (caution)
Diethylene Glycol
Dry Acetronitrile
Diethyl Ether
Procedure
Triphenylphosphine (5.03g, 18.8 mmol) was suspended in dry acetonitrile (20 ml) and cooled to 0°C in a 250 ml round-bottomed shlenck, using an Argon-vacuum regime. Bromine (0.96 ml, 18.8 mmol) was added dropwise to the reaction mixture, allowing the solution to become colourless after each portion was added, and was then allowed to warm to ambient temperature. Diethylene glycol (0.9 ml, 9.4 mmol) was added and the reaction heated at reflux (80°C) under dinitrogen for 18 h then allowed to cool to ambient temperature. The solvent was removed under reduced pressure (using using a pretrap). The resulting orange solid was stirred in diethyl ether (50 ml) for 1 h to give an orange solution and precipitate, which was filtered. The solvent was removed from the orange-coloured filtrate under reduced pressure to give an orange oil. This was further purified via Kügelrhor distillation to give a clear oil (bp 100°C under high vacuum). Yield: 1.45 g, 6.3 mmol, 67%.
Author Comments
Always use freshly recrystallised triphenylphosphine – recrystallised from hot hexane.
Conditions described in SyntheticPage [[474]] were modified since overnight reflux was required.
Kügelrhor distillation required to remove triphenylphosphine oxide by-product.
Dry acetonitrile was obtained by distillation from calcium hydride
Data
1H NMR (400 MHz, 298 K, CDCl3): δH 3.83 (t, 3JHH = 5.97 Hz, 2H, OCH2), 3.48 (t, 3JHH = 5.76 Hz, 2H, OCH2CH2).
13C {1H} NMR (101 MHz, 298 K, CDCl3): δC 70.96 (CH2, OCH2), 30.18 (CH2, OCH2CH2).
ESI-MS: m/z 72.4 [M-2Br]+.
IR v cm-1: 2966 w, 2856 w, 1739 m, 1438 w, 1421 m, 1361 w, 1279 m, 1226 w, 1111 s, 1030 m, 1005 m, 948 m, 726 m, 691 m, 663 m.
CHN (calculated for C4H8OBr2): C 20.78 % (20.72 %), H 3.49 % (3.48 %).
Lead Reference
R. Machinek and W. Luttke, Synthesis, 1975, 4, 255-256
Other References
Keywords
alkyl/alkenyl/aryl halides, bromination, ethers, substitution