Kröhnke pyridine synthesis
SyntheticPage 543
DOI:
Submitted: March 16, 2012, published: March 19, 2012
Authors
Suzanne Elizabeth Howson (s.e.howson@warwick.ac.uk)
A contribution from
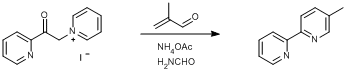
Chemicals
1-(2-Pyridylacetyl)pyridinium iodide (SyntheticPage [[541]])
Ammonium acetate (AnalaR)
Formamide (Sigma-Aldrich ‘Hydranal dry’ – put into ampoule, freeze-thaw degassed three times, and stored under argon)
Methacrolein (Sigma-Aldrich – Transferred from sure seal bottle to a small ampoule with side arm under argon. Freeze-thaw degassed three times. Vacuum transferred to a second ampoule using a trap-to-trap device fitted to a vacuum line – no heating required. Once vacuum transfer was complete, the ampoule was degassed once more and then stored under argon in the fridge)
Procedure
1-(2-Pyridylacetyl)pyridinium iodide (10.06 g, 30.85 mmol, 1.0 eq.) and ammonium acetate (5.47 g, 70.96 mmol, 2.3 eq.) were dissolved in formamide (100 ml). Freshly distilled methacrolein (2.38 g, 2.8 ml, 33.94 mmol, 1.1 eq.) was added via syringe. The reaction was stirred at 80°C for 6 hours and then cooled to ambient temperature. Water (80 ml) was added and the reaction mixture was extracted into diethyl ether (3 × 200 ml). The diethyl ether extracts were combined, dried over sodium sulphate and the solvent was removed under reduced pressure to leave the crude product as a yellow liquid. Column chromatography was carried out on silica eluting with DCM/methanol (20:1). The pure fractions were combined and the solvent was removed under reduced pressure. The product was then dissolved in DCM, filtered through celite to remove any silica and then the solvent was removed under reduced pressure. The resulting yellow liquid was dried overnight in vacuo at ambient temperature. Yield = 3.95 g, 23.21 mmol, 75%.
Author Comments
The prep from 'Synthesis, 1998, 321-324' extracts the crude reaction mixture first using diethyl ether as described in the procedure, and then using DCM. On doing this (and keeping the ether and DCM extracts separate) it was found that the DCM layer only contained impurities and no product, and so was discarded at this point.
Data
1H NMR (400 MHz, 298 K, CDCl3) δH 8.65 (1H, d, 3JHH = 5.0 Hz, Py), 8.50 (1H, d, 4JHH = 2.0 Hz, Py), 8.34 (1H, d, 3JHH = 8.0 Hz, Py), 8.27 (1H, d, 3JHH = 8.0 Hz, Py), 7.78 (1H, td, 3JHH = 7.5 Hz, 4JHH = 2.0 Hz, Py), 7.61 (1H, dd, 3JHH = 8.0 Hz, 4JHH = 2.0 Hz, Py), 7.28-7.24 (1H, m, Py), 2.38 (3H, s, CH3).
13C{1H} NMR (100 MHz, 298K, CDCl3) δC 156.41 (Py), 153.75 (Py), 149.75 (Py), 149.23 (Py), 137.58 (Py), 136.98 (Py), 133.53 (Py), 123.49 (Py), 120.91 (Py), 120.72 (Py), 18.46 (CH3).
MS (ESI) m/z 171.1 [M+H]+, 193.1 [M+Na]+.
Lead Reference
Synthesis, 1998, 321-324
Keywords
addition, aldehydes, alkenes, aromatics/arenes, heterocyclic compounds, ketones, Kröhnke, nucleophilic, ring formation, substitution