Large Scale reduction of Ketone with sodium borohydride
SyntheticPage 539
DOI:
Submitted: February 16, 2012, published: March 14, 2012
Authors
Ramesha Ramakrishna (ramesha63@hotmail.com)
A contribution from
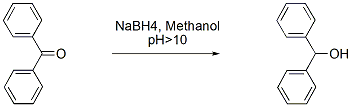
Chemicals
Methanol, commercial
Sodium borohydride , commercial
Procedure
Author Comments
1. Sodium borohydride is relatively stable in aqueous solution if the pH is above 10. Therefore it is possible to use all the hydrogen if the reaction medium is maintained alkaline.
2. Most literature procedure are done at either neutral or acidic conditions. Therefore more than 0.5 molar equivalent sodiumborohydride is used for the conversion. Because of this during workup there is large amount of hydrogen is liberated and it also poses safety risk.
3. For much larger scale, it is preferable to use nitrogen atmosphere during the reaction.
4. This is a practical method which can be used for any ketones/carbonyl compound
Data
1H NMR (300 MHz): 7.50-7.20 (m, 10H), 5.83 (s, 1H), 2.20 (s,OH)
13C NMR (75 MHz): 142.00, 128.25, 127.30, 127.12, 79.92
Lead Reference
Keywords
alcohols, aromatics/arenes, benzhydrol, benzophenone, ketones, reduction, sodiumborohydride