Esterification and Ritter reaction in one pot of cyanoacetic acid
SyntheticPage 532
DOI:
Submitted: January 21, 2012, published: January 23, 2012
Authors
Ramesha Ramakrishna (ramesha63@hotmail.com)
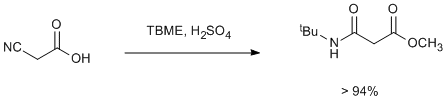
Chemicals
Cyanoacetic acid - commercial, 98% pure
Tert butyl methyl ether , commercial 98% pure
Concentrated sulfuric acid, Lab reagent, SD Fine Chem.
Procedure
To a well-stirred mixture of cyanoacetic acid (80 mmol) in tert butyl methyl ether (TBME, 50 mL) at about 10ºC was added sulfuric acid (18 g, 10 mL) in 10 min time. The mixture was then stirred at room temperature for 10–12 h. The reaction mixture was added to 20% sodium carbonate solution (100 mL). The reaction mass was extracted in TBME or dichloromethane (50 mL × 2). The combined organic layer was washed with water (100 mL), dried over sodium sulfate and concentrated under reduced pressure to obtain the product (94–98%). Most examples gave NMR pure product. This can be scaled up to 1 kg batches.
Author Comments
Water formed in the esterification is consumed in the ritter reaction to give almost quantitative addition of tert-butyl methyl ether.
Data
1H NMR ( CDCl3; 400MHz ):- δ 7.29 ( s, 1H, NH ), 3.74 ( s, 3H, OCH3 ), 1.36 ( s, 9H, -(CH3)3); 13C NMR ( CDCl3; 100 MHz):- 170.0, 163.8, 52.1, 51.2, 41.9, 28.4.
Lead Reference
One pot esterification and Ritter reaction: Chemo and regioselectivity from tert-butyl methyl ether.
Tetrahedron Lett, 2011, 52, 4262-4265
Other References
Keywords
addition, amides, carboxylic acids, electrophilic, esters, Ritter reaction, TBME, tert butyl methyl ether