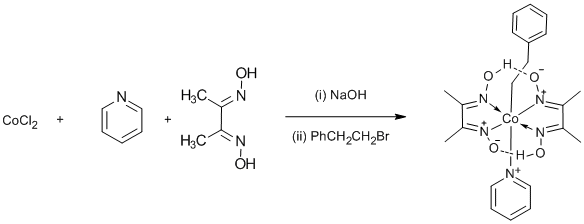
The one-pot preparation of a cobaloxime
SyntheticPage 531
DOI:
Submitted: January 20, 2012, published: January 23, 2012
Authors
Christopher Cooksey (rsc@chriscooksey.demon.co.uk)
Chemicals
Cobalt chloride hexahydrate
Pyridine
Dimethylglyoxime (2,3-Butanedione dioxime)
2-Bromoethylbenzene
Sodium hydroxide
MethanolProcedure
Author Comments
Crucial to the success of this reaction is the rigorous exclusion of oxygen before the addition of the halide. After the addition of 2 mols of NaOH, the formation of the cobaloxime(II) is complete. Further addition of NaOH causes the disproportionation of the cobaloxime(II) to cobaloxime(I) (blue-black) and cobaloxime(III) (brown, but not seen). If the intense blue-black cobaloxime(I) is not observed, then it is best to start again. In this method, half of the cobalt is lost, but if desired, all can be incorporated by adding NaBH4. The rate of reaction of cobaloxime(I) with halides is said to be 107 times faster than that of methoxide, leading to the suggestion that cobaloxime(I) is a super-nucleophile, but more likely it is a radical reaction. The reaction is successful for a wide range of halides or tosylates, even in the hands of inexperienced chemists (6th form pupils), giving typical yields of 60-80%. For observing the 1H NMR spectrum, it is advisable to filter the CDCl3 solution through flash silica gel to completely remove any paramagnetic Co(II) species. In this example, the 1H NMR resonance of the two dimethylglyoxime OH groups were not observed; they normally appear as a broad s at δH 18 ppm.
Data
δH (CDCl3) 1.80t (2H, J 7.4), 2.11s (12H), 2.20t (2H, J 7.4), 7.11-7.22m (5H), 7.33 ca. t (2H, J 7.6), 7.72tt (1H, J 7.6, 1.5), 8.61d (2H, J 7.6).
Found C 52.63; H 5.96; N 14.60, calc. for C21H28N5O4Co C 53.28; H 5.96; N 14.79%.
Lead Reference
Other References
[4-NitrobenzylCo(dmgH)2py] Brown TM, Cooksey CJ, Dronsfield AT and Crich D, J. Chem. Educ., 1990, 67(11): 973-974; DOI: 10.1021/ed067p973
[CH3CH2Co(dmgH)2py] Brown TM, Cooksey CJ, Dronsfield AT, Educ. Chem. 1990, 27(2): 45-47.
[n-C10H21Co(dmgH)2py] Brown TM and Cooksey CJ, Educ. Chem. 1987, 24(3): 77-80.
Keywords
organometallics