t-Butylperoxymercuration of cyclohexene
SyntheticPage 528
DOI:
Submitted: January 11, 2012, published: January 16, 2012
Authors
Christopher Cooksey (rsc@chriscooksey.demon.co.uk)
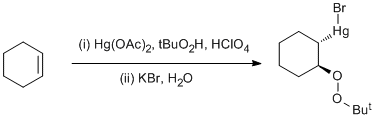
Chemicals
Cyclohexene
Mercury(II) acetate
t-Butyl hydroperoxide
Perchloric acid (60%)
Potassium bromideProcedure
t-Butyl hydroperoxide solution
MgSO4 (8 g) was added to a mixture of t-butyl hydroperoxide (10 g, 70%, 78 mmol) and CH2Cl2 (40 cm3) and the mixture stirred for 10 m. The drying agent was filtered and washed with CH2Cl2 (10 cm3) to give a solution of anhydrous t-butyl hydroperoxide.
Perchloric acid solution
A mixture of perchloric acid (2.0 g, 65%, 13 mmol) and CH2Cl2 (20 cm3) was magnetically stirred and cooled in ice. Acetic anhydride was slowly added until a homogeneous solution formed.
To mercury(II) acetate (6.4 g, 20 mmol) was added the t-butyl hydroperoxide solution followed by the perchloric acid solution. With magnetic stirring, cyclohexene (1.8 g, 22 mmol) dissolved in CH2Cl2 (10 cm3) was added. Most of the mercury(II) acetate rapidly dissolved except for some lumps which dissolved after 15 m. Water (100 cm3) was added and the organic phase separated and stirred with a solution of potassium bromide (5 g) in water (15 cm3) for 0.5 h. Water (100 cm3) was added, the organic phase separated, dried (MgSO4), and evaporated to give a white solid (9.9 g) after drying at 0.01 mm. Crystallisation from petrol (40/60, 75 cm3) gave bromo-(2-t-butylperoxycyclohexyl)mercury as white needles (7.32 g, 81%).
Author Comments
The reaction proceeds via nucleophilic attack of tBuOOH on an intermediate mercurinium ion. Competing reactions of attack by acetic acid or acetate leading to acetoxymercuration or by water leading to hydroxymercuration are minimised by using strong acid catalysis and an excess of tBuOOH, consequently chromatographic separation of the products is avoided.
CAUTION
Perchlorates and peroxides are potentially explosive. Please follow your local rules.
Organomercury compounds are very toxic
Data
δC (CDCl3) 24.56t, 26.58q (3C), 28.28t, 31.63t [J(199Hg-13C) 305 Hz], 32.75t [J(199Hg-13C) 756 Hz], 57.32d, 80.89s, 85.73d [J(199Hg-13C) 112 Hz].
δHg (CDCl3) –1199.6.
Lead Reference
Bloodworth AJ and Courtneidge JL, J. Chem. Soc., Perkin Trans. 1, 1981, 3258-3264
DOI: 10.1039/P19810003258
Keywords
addition, alkenes, nucleophilic, organometallics, peroxymercuration