Oxidative dehydration of aryl substituted succinic acids with selenium dioxide
SyntheticPage 525
DOI:
Submitted: December 22, 2011, published: January 5, 2012
Authors
John MacMillan (john.macmillan@temple.edu)
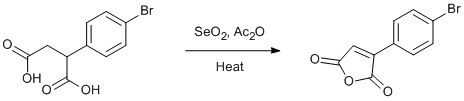
Chemicals
Selenium Dioxide (Alfa Inorganics)
Acetic Anhydride (Sigma Aldrich)
Diethyl Ether (Sigma Aldrich)
n-Hexane (Sigma Aldrich)
Procedure
A 250 ml round bottom flask with heating mantle, magnetic stirrer and water condenser was charged with 8.0 g (0.0293 mol) 3-bromophenyl succinic acid, 3.5 g (0.0310 mol) selenium dioxide and 70 ml acetic anhydride. A rubber tube ran from the top of the water condenser to a 1 l beaker charged with 500 ml commercial Chlorox. The mixture was refluxed for 22 h, the reduced selenium dioxide salts were filtered off with a water aspirator and sintered glass funnel. The filtrate was concentrated to 40 ml on a rotovap in hot 80ºC water. Cooling in ice to 0º C gave a copious tan precipitate, which was suction filtered a sintered glass funnel under water aspirator pressure. The filtrate was washed three times with 5 ml portions of 50/50 ether/hexane. Drying in a vacuum desicator blue Drierite desicant under pump vacuum (~2 torr) for 4 h gave 4.75 g (64.2%) 4-Bromophenyl maleic anhydride, as tan crystals. The product is suitable for futher reactions at this point but still has a slight odor. Recrystalization from hot hexane yieldded 2.82g of odor free product, Other runs gave yields in the 60-70% range.
Author Comments
Caution! Selenium dioxde is toxic. Wear Latex gloves. The reaction emits odiforous by-products and must be run in an efficient fume hood. While the exact stucture of these by-products is unknown, it must also be assumed that they are toxic selenium compounds. The odiforous emanations should be trapped in commercial Chlorox or other efficient bleaching agent.
This reaction is general, and was utilized to synthesize many other aryl-subsituted maleic anhydrides; see: http://furlip.solidwebhost.com/Additional-Arylmaleic-Anhydride-Syntheses.htm. These products are precursors for the synthesis of 4-and 5- aryl-substituted 1,3(3H) oxazine-2,6-diones (Oxauracils), see Other References.
Data
mp 152-154ºC
1H NMR: (60 Mhz, DMSO-D6), δ 7.8 ( 2H, d, J = 9 Hz, aromatics), 7.4 ( 2H, d, J = 9 Hz, aromatics) , 7.0 (s, 1H, olefinic).
IR (CDCl3), 1860(m), 1820(m),1800(m), 1775(vs), 1620(m), 1405(m), 1320(w), 1300(w), 1250(w), 1230(w), 1185(w), 1160(w), 1090(m), 1070(m), 1050(m), 1005(m), 820(s) cm-1.
Lead Reference
Richard K. Hill "A convenient Synthesis of Aryl Maleic Anhydrides", J. Org. Chem, 1961, 26(11), pp 4745-4747, DOI:10.10212/Jo0106a549 .
Other References
John H. MacMillan and Stephen S. Washburne "Further Investigation of the Interaction of Trimethylsilyl Azide with Substituted Maleic Anhydrides, Synthesis of 4-and 5-Aryl Substituted 1,3(3H) Oxazine-2,6-Diones" J.Heterocyclic Chemistry Vol. 12, p1215 (1975). DOI: 10.1002/jhet.5570120624
Keywords
anhydride, aromatics/arenes, dehydration, elimination, esters, heterocyclic compounds, oxidation