Addition of lithium 2-(methylsulfonyl)benzo[d]thiazole to benzoyl chloride
SyntheticPage 523
DOI:
Submitted: December 15, 2011, published: December 18, 2011
Authors
Jiri Pospisil (jiri.pospisil@uclouvain.be)
A contribution from
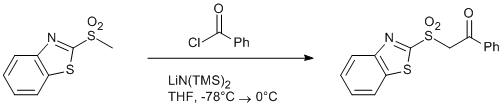
Chemicals
Benzoyl chloride, ACS reagent, 99% (Sigma-Aldrich)
LiN(TMS)2, 1.0 M solution in methyl tert-butyl ether, AcroSeal® (Acros Organics)
THF, ReagentPlus®, ≥99% (Sigma-Aldrich), freshly distilled from Sodium (Riedel-deHaën)Procedure
A solution of sulfone (5.0g, 23.4 mmol, 1.0 equiv) in dry THF (100 mL) was cooled to -78°C. LiHMDS (1.0 M solution in methyl tert-butyl ether) (52 mL, 52 mmol, 2.2 equiv) was placed into separated 100 mL flask and cooled to -78°C. Resulting cold solution of LiN(TMS)2 was then transferred into the sulfone solution using Teflon® cannula (øint. = 1 mm) within 1.5 min. Immediately after, precooled (-78°C) solution of benzoyl chloride (3.0 mL, 25.8 mmol, 1.1 equiv) in dry THF (15 mL) was added, again via Teflon® cannula.
The reaction mixture colour turned from slightly yellow to orange/red during the LiN(TMS)2 addition. Upon the addition of benzoyl chloride, however, the orange/red color of the reaction mixture started to fade and disappeared completely within a few minutes to leave yellowish solution. The resulting mixture was stirred at -78°C for additional 1h, allowed to warm to 0°C within 1h and stirred at 0°C for a further 30 min before saturated aqueous solution of NH4Cl (150 mL) was added. EtOAc (50 mL) was added into the reaction mixture and the resulting two phases were separated. The aqueous phase was extracted with EtOAc (3x250 mL). The combined organic layers were washed with brine (200 mL), dried over MgSO4, filtered and the solvents removed under reduced pressure. The residue was purified using flash column chromatography (SiO2, Petroleum ether:EtOAc = 4:1->2:1->1:1) yielding 7.1 g (95%) of a colorless solid.Author Comments
In my hands, the yields of this particular reaction were in a range of 90 and 97% (12 experiments).
A 500 mL round-bottom triple-neck flask was used and the cannulas were placed prior the addition of any of the reagents was started. It is important that benzoyl chloride solution is added immediately after the base. Extended time delays between the base and electrophile additions cause the lowering of reaction yield.
Both reagent solutions (LiN(TMS)2 and benzoyl chloride) must be precooled to -78°C prior their addition. This is to minimize the undesired homodimerization of starting sulfone (for more informations see: Pospíšil, J.; Robiette, R.; Sato, H.; Debrus, K. Org. Biomol. Chem., 2011, Accepted Manuscript, DOI: 10.1039/C1OB06510F).Data
1H-NMR (300 MHz, CDCl3): d = 5.22 (s, 2H), 7.43 – 7.53 (m, 2H), 7.55 – 7.69 (m, 3H), 7.94 (dd, J = 8.4, 1.2 Hz, 2H), 8.01 (dd, J = 7.0, 2.2 Hz, 1H), 8.20 (dd, J = 7.2, 2.1 Hz, 1H).
13C-NMR (75 MHz, CDCl3): d = 61.4, 122.6, 125.7, 127.9, 128.4, 129.17, 129.19, 134.9, 135.6, 137.3, 152.6, 165.5, 187.3.
IR (film): n-1 = 3063 (w), 2959 (w), 2925 (w), 2921 (w), 2919 (w), 1683 (s), 1598 (m), 1471 (m), 1338 (s), 1155 (s), 991 (m), 760 (s), 731 (s), 688 (s).
MS (APCI), m/z (%): 318 (100) [M++1], 319 (20), 236 (9), 105 (11).
El. an. for C15H11NO3S2, calc. C 56.76, H 3.49, N 4.41; found C 56.78, H 3.11, N 4.67.
Lead Reference
Other References
Supplementary Information
Keywords
addition, heterocyclic compounds, ketones, ketosulfones