Sonogashira Coupling of 4-Iodoanisole to Propargyl Alcohol
SyntheticPage 507
DOI:
Submitted: September 24, 2011, published: September 28, 2011
Authors
Christopher Kelly (christopher.b.kelly@uconn.edu)
A contribution from
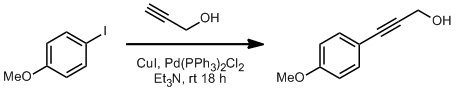
Chemicals
4-Iodoanisole (98%, Purchased from Sigma-Aldrich)
Propargyl Alcohol (99%, Purchased from Sigma-Aldrich)
Copper(I) Iodide (99.5% Purum, Purchased from Sigma-Aldrich)
Bis(triphenylphosphine)palladium(II) dichloride (> 99% Pure, Purchased from Sigma-Aldrich)
Triethylamine (99%, Purchased from ACROS)
Propargyl Alcohol (99%, Purchased from Sigma-Aldrich)
Copper(I) Iodide (99.5% Purum, Purchased from Sigma-Aldrich)
Bis(triphenylphosphine)palladium(II) dichloride (> 99% Pure, Purchased from Sigma-Aldrich)
Triethylamine (99%, Purchased from ACROS)
Procedure
To a 250 mL round bottom flask equipped with stirbar was added 4-iodoanisole, ( 4.68 g, 0.020 mol, 1 equiv.) and Et3N (150 mL, 0.134 M in the iodide). The solution was placed under a nitrogen atmosphere via a gas inlet adapter. To this solution was added PdCl2(PPh3)2 (0.702 g, 0.001 mol, 0.05 equiv.) and CuI (0.191 g, 0.001 mol, 0.05 equiv) all at once turning the solution yellow. Propargyl alcohol (1.46 g, 0.026 mol, 1.3 equiv) was then added all at once, turing the solution a cloudy green. The solution was stirred for 18 hours at room temperature and monitored by TLC (8:2 Hex: EtOAc). Upon completion of this time, the solution was filtered through Celite®, eluting with ethyl acetate. The filterate was then further diluted with EtOAc (≈50 mL) and deionized water (≈100 mL) and the layers were seperated. The aqueous layer was extracted three times with EtOAc. The combine organic layers were dried with Na2SO4 and the solvent was removed in vacuo yielding a thick dark liquid. Purification was accomplished by flash column chromoatgraphy (Gradient 9:1 Hex: EtOAc to 8:2) affording pure 6r as a tan powdery solid (2.75 g, 85% yield).
Data
1H NMR (CDCl3, 300 MHz) d ppm 1.65 (t, J = 6.14 Hz, 1 H) 3.81 (s, 3 H) 4.48 (d, J = 6.14 Hz, 2 H) 6.84 (d, J = 9.10 Hz, 2 H) 7.38 (d, J = 8.77 Hz, 2 H) 13C NMR (CDCl3, 101 MHz) d ppm 51.85 (CH2) 55.53 (CH3) 85.82 (C) 86.22 (C) 114.21 (CH) 114.91 (C) 133.44 (CH) 159.95 (C)
Lead Reference
Belot, A.; Vogt, K. A.; Besnard, C.; Krause, N.; Alexakis, A. Angew. Chem. Int . Ed. 2009 , 48 , 8923.
Keywords
alcohols, alkyl/alkenyl/aryl halides, alkynes, aromatics/arenes, Bis(triphenylphosphine)palladium(II) dichloride, Copper(I) Iodide, organometallics, oxidative addition, reductive elimination, transition metal catalysed