Wittig Reaction
SyntheticPage 506
DOI:
Submitted: September 14, 2011, published: September 14, 2011
Authors
Anish Mistry (a.mistry@warwick.ac.uk)
A contribution from
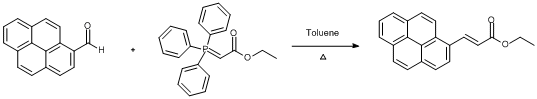
Chemicals
Ethyl (triphenylphosphoranyliden)acetate (1 equiv, prepared)
1-pyrenecarboxaldehyde (1 equiv, purchased from Sigma-Aldrich)
Toluene
Chloroform
Petroleum ether (40 - 60 oC)
1-pyrenecarboxaldehyde (1 equiv, purchased from Sigma-Aldrich)
Toluene
Chloroform
Petroleum ether (40 - 60 oC)
Procedure
1-pyrenecarboxaldehyde (1.13 g, 4.9 mmol) was added to a solution of ethyl (triphenylphosphoranyliden)acetate (1.71 g, 4.9 mmol) in toluene (80 ml) and the reaction mixture heated under reflux for approximately 96 hours. The mixture was left to cool and solvent removed in vacuo to form a crude yellow/orange solid. The crude product was purified by coloumn chromatography using (8:2) chloroform : petroleum ether (40 - 60 oC) to yield yellow granules (1.3 g, 89%).
Data
δH(400MHz, CDCl3) ppm: 1.44 (3H, t, J 7.0, CH3), 4.40 (2H, q, J 7.0, CH2), 6.75 (1H, d, J 15.5, CH alkene), 8.04 - 8.36 (8H, m, aryl), 8.52 (1H, d, J 9.5, aryl), 8.88 (1H, d, J 15.5, CH alkene)
Lead Reference
Ta-Hsien Chuang, Shiow-Ju Lee, Cheng-Wei Yang and Pei-Lin Wu, Expedient synthesis and structure–activity relationships of phenanthroindolizidine and phenanthroquinolizidine alkaloids, Org. Biomol. Chem., 2006, 4, 860–867 http://dx.doi.org/10.1039/b516152e
Keywords
aldehydes, alkenes, aromatics/arenes, phosphorus ylide, Wittig