Biginelli reaction with benzaldehyde
SyntheticPage 500
DOI:
Submitted: August 1, 2011, published: August 1, 2011
Authors
Sirin Gülten (siringulten@hotmail.com)
A contribution from
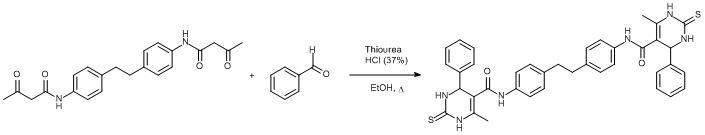
Chemicals
3-Oxo-N-[4-[4-(3-oxobutanoylamino)phenyl]ethylphenyl]butanamide Gulten, S, SyntheticPages, 2011, 498
Benzaldehyde (Acros Organics)
Thiourea (Acros Organics)
37% HCl (Merck)
Procedure
A mixture of 3-oxo-N-[4-[4-(3-oxobutanoylamino)phenyl]ethylphenyl]butanamide (300 mg, 0.789 mmol), thiourea (183 mg, 2.41 mmol), benzaldehyde (0.24 mL, 2.41 mmol) and catalytic amount of conc. HCl (4 drops) in EtOH (10 mL) was refluxed overnight. After the completion of reaction, the reaction mixture was allowed to cool. The solid product formed was filtered, washed with water (5 mL) and cold ether (3x5 mL) to remove the unreacted thiourea or benzaldehyde or 3-oxo-N-[4-[4-(3-oxobutanoylamino)phenyl]ethylphenyl]butanamide and dried. The title compound was obtained as a cream coloured solid (450 mg, 88% yield).
Author Comments
This reaction was carried out with catalytic amount of pTSA to give the corresponding bis Biginelli product in good yield.
This reaction was carried out by Elif Özdemir in the final year research project under my supervision.
Data
IR-ATR: 3377, 3188, 3031, 2969, 2922, 2858, 1672, 1632, 1593, 1515, 1180, 824 cm-1.
1H NMR (400 MHz, DMSO-d6): ppm = 2.06 (s, 6H, CH3), 2.76 (s, 4H, CH2), 5.39 (s, 2H, CH) 7.08 (d, 4H, J= 7.8 Hz, CHAr), 7.25-7.40 (m, 10H, CHAr), 7.43 (d, 4H, J= 7.8 Hz, CHAr), 9.41 (s, 2H, NH), 9.65 (s, 2H, NH), 9.97 (s, 2H, NH).
13C NMR (100 MHz, DMSO-d6): ppm = 174.10, 164.74, 143.04, 136.75, 136.48, 135.23, 128.58, 128.38, 127.63, 126.33, 119.62, 107.29, 55.06, 36.47, 16.45.
Lead Reference
Vijay Virsodia, Raghuvir R.S. Pissurlenkar, Dinesh Manvar, Chintan Dholakia, Priti Adlakha, Anamik Shah, Evans C. Coutinho, Synthesis, screening for antitubercular activity and 3D-QSAR studies of substituted N-phenyl-6-methyl-2-oxo-4-phenyl-1,2,3,4-tetrahydro-pyrimidine-5-carboxamides, Eur. J. Med. Chem., 43, 2008, 2103-2115. doi:10.1016/j.ejmech.2007.08.004
Other References
B.R. Prashantha Kumar, Gopu Sankar, R.B. Nasir Baig, Srinivasan Chandrashekaran, Novel Biginelli dihydropyrimidines with potential anticancer activity: A parallel synthesis and CoMSIA study, Eur. J. Med. Chem., 44, 2009, 4192-4198. doi:10.1016/j.ejmech.2009.05.014
Keywords
addition, amides, aromatics/arenes, Biginelli dihydropyrimidine, heterocyclic compounds, nucleophilic, thermal