Selective Monophosphorylation of ethylenediamine
SyntheticPage 491
DOI:
Submitted: March 31, 2011, published: April 1, 2011
Authors
Leandro Pedrosa (leandropedrosa@globo.com)
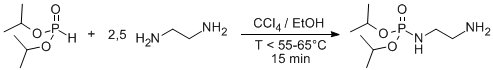
Chemicals
ethylenediamine
ethanol
diisopropyl phosphonate
carbon tetrachloride
Procedure
A solution of the amine (45 mmol, 2.5 eq) in ethanol (9 mL) was added of recently distilled diisopropyl phosphonate (18 mmol, 1 eq) in carbon tetrachloride (4 mL) and ethanol (5 mL) dropwise. The temperature should not exceed 55–65°C during the addition. The mixture was stirred for 15 min until diisopropyl phosphonate wasn´t detected by TLC. The solution was evaporated under reduced pressure. Water (50 mL) was added to the residue, and the product was extracted twice with 20 mL portions of CH2Cl2; the organic phase was dried over MgSO4 and evaporated giving a pale yellow oil (2.02 g, 50%) product of high purity. Additional washes with water can be performed if necessary.
Author Comments
A 2.5-fold excess of diamine must be used to neutralize HCl and still keep a basic pH necessary to catalyze the reaction. The addition of phosphonate to the amine should not exceed 10 min nor overtake the range 55–65°C; otherwise, bis-phosphorylation will occur preferentially. This particular reaction protocol can be applied to different aliphatic diamines ranging from the stated ethylenediamine up to 1,6-diaminohexane.
Data
1H NMR (300 MHz, CDCl3) 1.31, 1.32 (12H, 2d, CH3, JHCCH = 6.1); 2.55 (2H, s, NH2 broad); 2.84 (2H, t, CH2NH2, JHCCH = 5.5); 2.99 (2H, m, CH2NHP); 3.31 (1H, m, NH-P); 4.59 (2H, dhep, HCO, JHCCH = 6.2, JPOCH = 7.6)
13C NMR (75.42 MHz, CDCl3) 23.75 (2d, CH3, JCCOP = 4.6); 42.71 (d, CH2CH2NP, JCCNP = 5.7); 43.68 (CH2NP); 70.79 (d, CH, JCOP = 5.7)
31P NMR (121.42 MHz, CDCl3) 7.73
Lead Reference
Other References
T. S. Torres, W. P. de Macedo, L. F. Pedrosa, M. C. B. V. de Souza, V. F. Ferreira, A. C. Cunha, T. Fogel, F. C. Santos, I. P. Marques, I. C. P. P. Frugulhetti, M. C. de Souza, Lett.Org. Chem. 2008, 5, 644. doi:10.2174/157017808786857615
Supplementary Information
Keywords
amines, organo phosphorous