Intramolecular nitrile oxide cyclization (INOC). Part II
SyntheticPage 487
DOI:
Submitted: February 14, 2011, published: February 14, 2011
Authors
Sirin Gülten (siringulten@hotmail.com)
A contribution from
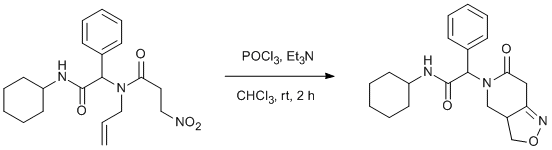
Chemicals
N-allyl-N-(2-(cyclohexylamino)-2-oxo-1-phenylethyl)-3-nitropropanamide Gulten, S, SyntheticPages, 2011, 485
POCl3 (Acros Organics)
Et3N (Merck)
CHCl3
CH2Cl2
MgSO4
Procedure
A solution of POCl3 (1.00 mmol, 0.093 mL) in CHCl3 (3.5 mL) was added dropwise to a mixture of N-allyl-N-(2-(cyclohexylamino)-2-oxo-1-phenylethyl)-3-nitropropanamide (0.670 mmol, 250 mg) and Et3N (2.68 mmol, 0.373 mL) in CHCl3 (3.5 mL). After 30 min Et3N (1.33 mmol, 0.186 mL) POCl3 (0.499 mmol, 0.0465 mL) in CHCl3 (1.5 mL) were added and the reaction mixture was stirred for 2 h at room temperature under nitrogen atmosphere at which point water (20 mL) was added. The crude product was extracted with CH2Cl2 (3 x 15 mL) and dried over anhydrous MgSO4. After evaporation of the organic solvent, the crude product was purified by flash chromatography using silica gel (eluent: ethyl acetate-hexane; 1 : 1) to obtain title compound as a white solid (168 mg, 71% yield, mp 192-194 °C, Rf= 0.20 (ethyl acetate-hexane; 1 : 1). 50:50 Mixture of diastereoisomers as determined by 1H-NMR integration.
Author Comments
The author thanks Çanakkale Onsekiz Mart University (BAP 2010/109) for financial support.
Data
IR-ATR: 3255, 3086, 2936, 2922, 2853, 1660, 1640, 1557 cm-1
1H NMR (400 MHz, DMSO-d6): ppm = 8.27 (1H, d, NH, J= 7.77 Hz), 7.43-7.33 (3H, m, aromatic CH), 7.23-7.20 (2H, m, aromatic CH), 6.29 (0.5H, s, CH-Ar), 6.22 (0.5H, s, CH-Ar), 4.34-4.27 (1H, m), 3.87-3.83 (1H, skew dd, CH2O), 3.81-3.77 (1H, skew t, CH2O), 3.71 (1H, m), 3.65-3.59 (2H, m), 3.57-3.51 (2H, m), 1.80-1.67 (4H, m, cyclohexyl CH2), 1.57-1.54 (1H, m, cyclohexyl CH2), 1.30-1.09 (5H, m, cyclohexyl CH2).
13C NMR (100 MHz, DMSO-d6): ppm = 167.86, 167.81, 166.38, 166.17, 155.47, 155.41, 135.92, 135.70, 128.74, 128.63, 128.60, 128.41, 127.95, 127.86, 70.31, 70.10, 59.76, 58.43, 47.72, 47.17, 46.31, 46.02, 45.84, 32.24, 32.12, 32.08, 32.06, 30.99, 25.11, 24.53, 24.44.
Lead Reference
Other References
1. Loubinoux, B.; Gerardin, P.; Schneider, R.; Ahrach, M.; Boëlle, J. Trends Heterocycl. Chem. 1995, 4, 135.
2. Chiacchio, U.; Corsaro A.; Librando, V.; Rescifina, A.; Romeo, R.; Romeo, G. Tetrahedron, 1996, 52, 14323
Keywords
addition, amides, aromatics/arenes, heterocyclic compounds, intramolecular nitrile oxide cyclization (INOC), nucleophilic