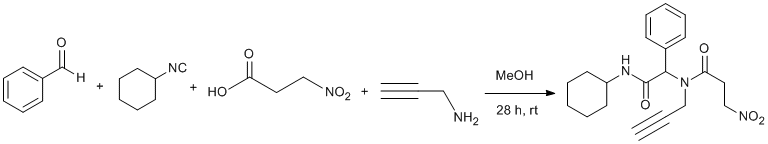
Ugi Reaction. Part I
SyntheticPage 484
DOI:
Submitted: February 11, 2011, published: February 11, 2011
Authors
sirin gulten (siringulten@hotmail.com)
Chemicals
Benzaldehyde (Acros Organics)
Propargylamine (Acros Organics)
Cyclohexyl isocyanide (Merck)
3-Nitropropionic acid (Sigma)
MeOH
Procedure
A solution of benzaldehyde (1.70 mmol, 0.172 mL), propargylamine (1.70 mmol, 0.11 mL), cyclohexyl isocyanide (1.70 mmol, 0.211 mL) and 3-nitropropionic acid (1.70 mmol, 0.202 g) in 10 mL MeOH was stirred at room temperature under nitrogen atmosphere. After 28 h the solution was concentrated under reduced pressure to leave a yellow solid. The residue was washed with cold Et2O (3x20 mL) to give pure title compound as pale yellow solid (480 mg, 76% yield, mp 179-180 °C, Rf= 0.52 (ethyl acetate-hexane; 1 : 1). 1H and 13C NMR spectra showed title compound is a mixture of two rotamers in an approximately 1:3.5 ratio.
Author Comments
The Ugi multicomponent reaction proceeded cleanly from starting materials. It has found numerous applications in organic chemistry.
The author thanks Çanakkale Onsekiz Mart University (BAP 2010/109) for financial support.
Data
IR-ATR: 3257, 3086, 2926, 2855, 2122, 1639, 1631, 1564 cm-1.
1H NMR (400 MHz, DMSO-d6): (both rotamers) ppm = 8.34 (0.22H, d, NH, J= 7.62 Hz), 8.21 (0.78H, d, NH, J= 7.63 Hz), 7.44-7.32 (3H, m, aromatic CH), 7.23 (2H, d, aromatic CH, J= 7.03 Hz), 6.14 (0.78H, s, CH-Ar), 5.68 (0.22H, s, CH-Ar), 4.80 (1.56H, t, CH2-NO2, J= 5.37 Hz), 4.75 (0.44H, t, CH2-NO2, J= 5.37 Hz), 4.30 (1.56H, skew dq, CH2CH2-NO2), 4.07 (0.44H, skew q, CH2CH2-NO2), 3.62-3.57 (1H, m, CHNH), 3.28-3.25 (2H, m, CH2C≡CH), 3.02 (1H, s, CH2C≡CH), 1.77-1.63 (4H, m, cyclohexyl CH2), 1.56-1.53 (1H, m, cyclohexyl CH2), 1.30-1.07 (5H, m, cyclohexyl CH2).
13C NMR (100 MHz, DMSO-d6): (both rotamers) ppm = 170.51, 169.79, 168.26, 167.64, 136.27, 135.96, 129.18, 129.07, 128.95, 128.81, 128.65, 128.43, 81.00, 80.37, 74.42, 72.46, 70.88, 62.58, 59.41, 48.33, 48.17, 34.88, 34.28, 32.67, 32.53, 30.54, 25.60, 25.00, 24.91.
Lead Reference
Supplementary Information
Keywords
addition, amides, aromatics/arenes, nucleophilic, Ugi reaction