Bromination of an alcohol
SyntheticPage 474
DOI:
Submitted: December 6, 2010, published: December 6, 2010
Authors
Suzanne Elizabeth Howson (s.e.howson@warwick.ac.uk)
A contribution from
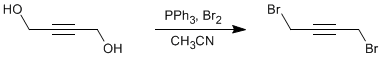
Chemicals
2-Butyne-1,4-diol
Bromine
Triphenylphosphine (recrystallised from hexane before use)
Dry Acetonitrile (dried by heating to reflux for 3 d under dinitrogen over calcium hydride)
Diethylether
Bromine
Triphenylphosphine (recrystallised from hexane before use)
Dry Acetonitrile (dried by heating to reflux for 3 d under dinitrogen over calcium hydride)
Diethylether
Procedure
Procedure
Triphenylphospine (6.25 g, 23.82 mmol, 2.05 eq.) was suspended in dry acetonitrile (20 ml) in a Schlenk and then cooled to 0°C using an ice-water bath. Bromine (1.2 ml, 3.71 g, 23.24 mmol, 2.00 eq.) was added dropwise using a syringe (after each drop the solution was allowed to return to colourless before the next drop was added). 2-Butyne-1,4-diol (1.00 g, 11.62 mmol, 1.00 eq.) was dissolved in dry acetonitrile (10 ml) and was slowly added to the reaction mixture (n.b. all solid triphenylphosphine is taken into solution at this point). The reaction was stirred overnight at ambient temperature under argon (n.b. as the reaction proceeds solid precipitates from the solution). The solvent was removed from the reaction mixture and the residue was taken up in diethylether (~150 ml). The solution was then filtered and the solvent was removed from the filtrate to leave the crude product as a yellow liquid. This crude product was purified by Kügelrohr distillation to give a clear liquid (bp 80°C under high vacuum). Purified yield = 1.64 g, 7.74 mmol, 67%.
Data
1H NMR (400 MHz, 298 K, CDCl3) δH 3.95 (4H, s, CH2).
13C{1H} NMR (100 MHz, 298 K, CDCl3) δC 81.6 (C≡C), 13.8 (CH2).
Lead Reference
R. Machinek and W. Luttke, Synthesis, 1975, 4, 255-256
Other References
G. Blond, C. Bour, B. Salem and J. Suffert, Organic Letters, 2008, 10, 1075-1078 (compound 72)
Keywords
alcohols, alkyl/alkenyl/aryl halides, alkynes, bromination, substitution