Olefination of 6-bromo-1H-indole-2,3-dione with 1H-indol-3-yl acetate via the 2-chloroindoleninone
SyntheticPage 465
DOI:
Submitted: August 17, 2010, published: August 24, 2010
Authors
Christopher Cooksey (rsc@chriscooksey.demon.co.uk)
A contribution from
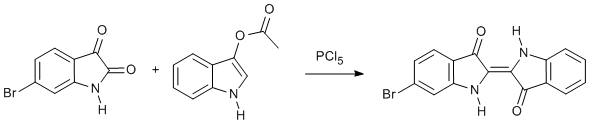
Chemicals
Procedure
Author Comments
Isatins react with PCl5 to give (supposedly) 2-chloroindoleninones. 6-Bromoisatin is reported (Baker JT, Duke CC, Aust J Chem 1976, 29, 1023 – 1030) to be unreactive to PCl5 in refluxing toluene and decomposition occurred with higher boiling point solvents. Using chlorobenzene appeared to be satisfactory but yields were modest. But the reaction is convenient and the product is easily isolated because it is insoluble in nearly all cold solvents. The lack of solubility precludes chromatographic separation of useful amounts of the product, but recrystallisation can be achieved from nearly any solvent with b.p. >200 °C. Recrystallisation of 6-bromoindigo (18.1 mg) from ethyl benzoate (10 cm3) gave 14.5 mg. NMR characterisation was via the orange N,N'-trifluoroacetate derivative. 6-Bromoindigo, isolated from natural shellfish purple, has more recently been directly characterised by NMR (http://www.imog2007.org/files/Wednesday%20Posters/Wednesday%20Analytical%20Developments/P277-WE%20James.pdf).
Heating to 130 °C for 1 h gave a lower yield (16%); the reverse reaction using isatin and 6-bromoindoxyl acetate gave an even lower yield (10%). Modest yields were obtained from the appropriate bromoisatin for other monobromoindigos: 4-bromo (28%), 5-bromo (38%), 7-bromo (27%).Data
EI-MS m/z (%) 342 (100), 340 (100), 314 (14), 312 (14), 262 (5), 233 (9), 205 (28)
λmax (tetrachloroethane) 601 nm
N,N'-trifluoroacetate:
δH (CDCl3) 8.30d (1H, 1.2), 8.06d (1H, 8.4), 7.95d (1H, 7.7), 7.79td (1H, 7.4, 1.3), 7.75d (1H, 8.1), 7.56dd (1H, 8.1, 1.4), 7.42t (1H, 7.4)
Lead Reference
Keywords
nucleophilic