Permanganate oxidation of a methyl group to carboxylic acid
SyntheticPage 455
DOI:
Submitted: August 2, 2010, published: August 3, 2010
Authors
Christopher Cooksey (rsc@chriscooksey.demon.co.uk)
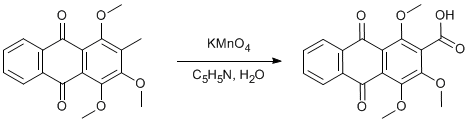
Chemicals
1,2,4-Trimethoxy-3-methyl-anthraquinone. page 443
KMnO4Procedure
1,2,4-Trimethoxy-3-methyl-anthraquinone (1.047 g, 3.4 mmol) was magnetically stirred in pyridine (3 cm3) and water (7 cm3) at 85 °C. KMnO4 (1.5 g, 9.5 mmol) was added over 0.5 h. Heating was continued for 6 h the mixture was allowed to stand 2 d at room temperature. The mixture was diluted with water (10 cm3) and diethyl ether – hexane (3:2, 50 cm3) and the solution filtered. The residue was washed with 10% aqueous pyridine (2 x 10 cm3) and the phases separated. The aqueous phase was acidified with H2SO4 to give yellow needles (269 mg, 23%) of the carboxylic acid.
Evaporation of the diethyl ether – hexane phase gave the starting material (561 mg, 54%).Author Comments
Other attempts using KMnO4 as oxidant gave poorer yields:
t-BuOH reflux 3h (0%) (89% recovered)
aq. C5H5N 50-55 °C 15 h (16%)(38% recovered)
C5H5N, Bu4N+Cl‾ rt 3 d (0%) (93% recovered)
NaOH, t-BuOH reflux 4 h (8%) (52% recovered)
Bu4N+MnO4‾ aq. C5H5N reflux 1 h (0%) (0% recovered)
After such persistent discouragement, an alternative route was found to be more successful: CH3 → CH2Br → COOHData
δH (CDCl3) 8.21m (2H), 7.78m (2H), 4.13s (3H), 4.06s (3H), 4.03 (3H)
EI-MS m/z (%), 342 (97), 312 (41), 310 (11), 309 (50), 298 (17), 297 (98)Lead Reference
Smith CW, Ambler SJ, Steggles DJ, Tet Lett, 1993, 34(46), 7447-7450
doi:10.1016/S0040-4039(00)60149-1Other References
Kreuchunas A, J Org Chem, 1956, 21(3), 368-369
DOI: 10.1021/jo01109a604Keywords
aromatics/arenes, carboxylic acids, oxidation