Acylation/cyclisation of a methoxyl-substituted benzoyl benzoic acid
SyntheticPage 443
DOI:
Submitted: July 22, 2010, published: July 23, 2010
Authors
Christopher Cooksey (rsc@chriscooksey.demon.co.uk)
A contribution from
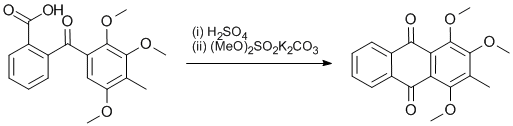
Chemicals
Procedure
A mixture of 2-(2,3,5-trimethoxy-4-methyl-benzoyl)-benzoic acid (15 g, 45.5 mmol) and concentrated sulfuric acid (100 cm3) was magnetically stirred on an oil bath at 50-52 ºC for 6 h. After standing overnight the dark red solution was poured on to ice and the deep orange-brown precipitate filtered and washed with water. After drying 11 g (83%) of partially demethylated product was obtained. The partially demethylated product (3.5 g) was heated with magnetic stirring under reflux with dimethyl sulfate (9 cm3), K2CO3 (12 g) and 2-butanone (50 cm3) for 6 h. The suspension was filtered and the residue washed with 2-butanone (2 x 20 cm3). Evaporation of the filtrate gave 1,2,4-trimethoxy-3-methyl-anthraquinone as yellow brown needles (3.5 g), mp. 122.2 ºC.
Author Comments
H2SO4 cyclisation of the methoxyl-substituted benzoyl benzoic acid gave a mixture of partially demethylated anthraquinones. Remethylation with (MeO)2SO2 and K2CO3 in 2-butanone gave yellow 1,2,4-trimethoxy-3-methyl-anthraquinone as the only product.
Reaction conditions of 150-160 ºC, 30 m reportedly lead to complete demethylation and a 5% yield of the trihydroxymethyl-anthraquinone (Raistrick H, Robinson R, Todd AR, J Chem Soc 1937, 80-88, doi: 10.1039/JR9370000080). Recommended conditions for similar reactions (Smith CW, Ambler SJ, Steggles DJ, Tet Lett 1993, 34(46), 7447-7450, doi:10.1016/S0040-4039(00)60149-1), 1 h ambient temperature, gave only recovered starting materials. Reaction conditions of 50-52 ºC for 6 h gave a 1:1 mixture of one red 3-methyl-2-methoxy-1,4-dihydroxy- and two yellow isomers 3-methyl-2,4-dimethoxy-1-hydroxy- and 3-methyl-1,2-dimethoxy-4-hydroxy- anthraquinones. 1,2,4-Trimethoxy-3-methyl-anthraquinone (2 g) was crystallised from hexane (60 cm3) to give yellow needles (1.48 g). [Hirose Y, Chem Pharm Bull, 1960, 8(5), 417-426, yellow needles, mp. 126-127 ºC (MeOH)].Data
δH (CDCl3) 8.19m (2H), 7.73m (2H), 3.99s (3H), 3.97s (3H), 3.92s (3H), 2.33s (3H)
Lead Reference
Raistrick H, Robinson R, Todd AR, J Chem Soc 1937, 80-88, doi: 10.1039/JR9370000080
Other References
Smith CW, Ambler SJ, Steggles DJ, Tet Lett 1993, 34(46), 7447-7450, doi:10.1016/S0040-4039(00)60149-1
Keywords
acylation, anthraquinone, carboxylic acids, cyclisation, methylation