Amidation of carboxylic acids using 2-Chloro-1-methylpyridinium iodide (Mukaiyama's reagent)
SyntheticPage 434
DOI:
Submitted: July 5, 2010, published: July 5, 2010
Authors
Marc Hutchby (chwmh@bristol.ac.uk)
A contribution from
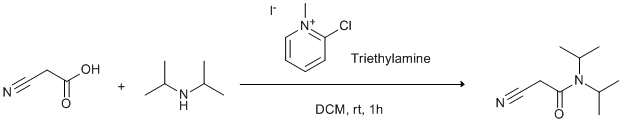
Chemicals
Cyanoacetic acid (Acros Organics)
2-Chloro-1-methylpyridinium iodide (Acros Organics)
Diisopropylamine (Acros Organics)
Triethylamine (Fisher Scientific)
DCM (anhydrous)
2-Chloro-1-methylpyridinium iodide (Acros Organics)
Diisopropylamine (Acros Organics)
Triethylamine (Fisher Scientific)
DCM (anhydrous)
Procedure
To a suspension of cyanoacetic acid (255 mg, 3 mmol) in anhydrous DCM (9 mL) was added 2-chloro-1-methylpyridinium iodide (2.3 g, 9 mmol). The solution became yellow in colour with copious amounts of a white precipitate. The solution was stirred under an inert atmosphere at room temperature for 5 minutes. To this was added diisopropylamine (0.42 mL, 3mmol) followed immediately by the addition of triethylamine (2.1 mL, 15mmol) over 1 minute. Both additions were performed at room temperature resulting in a slight exotherm. The solution became pale yellow in colour still with a large amount of a white precipitate. After 1 h the reaction was diluted with 15 mL 1M HCl and a further 10 mL of DCM. The organic layer was further washed with 2 X H2O (15 mL) and dried over MgSO4. The solvent was then removed under vacuum and the residue purified by column chromatography (40% EtOAc in Peteroleum Ether).
Author Comments
Due to the polar nature of the carboxylic acids used, it is often difficult to follow the reaction via TLC. However, most reactions sem to be complete after 1 h. The reaction is successful in amidating a range of carboxylic acids including mono alkyl malonates and 2-(phenylsulfonyl)acetic acid.The reaction also tolerates very bulky amine coupling partners. These include N-tert-butyl-N-ethylamine and N-tert-butylisopropylamine.
Data
1H NMR (400 MHz, CDCl3): 3.79 (septet, J = 6.6 Hz, 1H), 3.48 (septet, J = 6.7 Hz 1H), 3.47 (s, 2H), 1.38 (d, J = 6.8 Hz 6H), 1.25 (d, J = 6.6 Hz, 6H)
Keywords
Amidation, amides, carboxylic acids, nucleophilic
Comments
Hi Marc - very useful prep. Could you put in the yield you obtained?
By Kevin Booker-Milburn on July 9, 2010
Yield
The yield for this particular reaction is >99%.
The reaction proceeds with excellent yields (many of them quantitative) for all carboxylic acids I have tried.
In addition to this, increasing the steric bulk of the amine coupling partner has no detrimental effect on yield.
By Marc Hutchby on July 12, 2010
aniline
How useful is this reaction when the amine is an aniline? Also can one use 1,3-dione carboxylic acids?
By Hormoz on December 2, 2010
aniline
I have not used aniline myself but as the reaction is believed to go via a ketene, I would anticipate the reaction to work well.
If by 1,3-dione carboxylic acids you mean malonic acids then the reaction proceeds with good yields.
By Marc Hutchby on December 2, 2010
Acid scope
does this reaction work with the simple 3-Butynoic acid? What about the corresponding allenoic acid i.e.2,3-Butadienoic acid. These acids cannot be coupled using usual coupling reagents like HATU, HBTU, EDC etc.
By Ata Abbas on February 1, 2012
re: acid scope
I haven't specifically tried the acids you mentioned.
The reaction works extremely well with electron withdrawing groups, however I would anticipate the reaction would also work well with electron neutral and electron rich substrates, it just might take a little longer (perhaps overnight instead of 1 h). Pre-stirring the Mukiyamas reagent with the acid, prior to the addition of the amine/base may also help.
The type of amine coupling partner will also affect the reaction. Smaller amines like dimethylamine are always faster, giving higher yields.
By Marc Hutchby on February 2, 2012
Base
Does triethylamine act like a base here? Can excess of diisopropylamine be added in the beginning instead of adding TEA?
By Ruili Feng on November 26, 2014