Methanolysis of phthalic anhydride
SyntheticPage 428
DOI:
Submitted: July 1, 2010, published: July 5, 2010
Authors
Christopher Cooksey (rsc@chriscooksey.demon.co.uk)
A contribution from
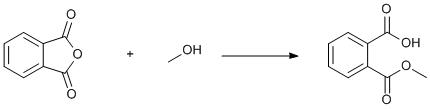
Chemicals
methanol
Procedure
Author Comments
A subsequent perusal of the literature reveals a much simpler procedure, 0.5 h reflux and no toluene: "Under a nitrogen atmosphere, a mixture of 75.0 grams (0.506 mole) of phthalic anhydride in 120.0 grams (3.75 moles) of methanol was stirred and heated under reflux for 30 minutes. During this time a complete solution was attained. The reaction mixture was concentrated under reduced pressure to a residual oil. The oil was placed in a refrigerator where it solidified to yield when dried, 93.0 grams of phthalic acid, mono-methyl ester; m.p. 80-81.5 ºC." in quantitative yield.
Chang Jun H, Baum J S, US 4892578 (9 Jan 1990)Data
Lead Reference
E L Eliel, A W Burgstahler, J Amer Chem Soc, 1949, 71, 2251-2252. doi: 10.1021/ja01174a509
Other References
Keywords
addition, alcohols, carboxylic acids, esters, nucleophilic