Alkylation of a phenol
SyntheticPage 412
DOI:
Submitted: May 27, 2010, published: May 27, 2010
Authors
David Fox (d.j.fox@warwick.ac.uk)
A contribution from
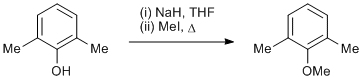
Chemicals
2,6-dimethylphenol
sodium hydride (60 % in mineral oil)
methyl iodide
sodium hydride (60 % in mineral oil)
methyl iodide
Procedure
Sodium hydride (60% in mineral oil) (44.0 g, 1.10 mol) was washed with petroleum ether (3 × 100mL) and then suspended in dry THF (500 ml) and the mixture was cooled to 0 oC. 2,6-Dimethylphenol (122 g, 1.00 mol) was then added portion-wise and the reaction was stirred for 30 min. Methyl iodide (213 g, 1.50 mol) was then added and the reaction heated at reflux for 18 h. The mixture was allowed to cool and the solvent removed in vacuo. The residue was partitioned between water (500 ml) and CH2Cl2 (3 × 500mL). The combined organic layers were dried (MgSO4) and reduced in vacuo to give a yellow oil. This oil was distilled at atmospheric pressure to afford 2,6-dimethylanisole as a colourless oil (97.9 g, 72%).
Data
bp 182 oC (ref 181-182 oC); δH (200MHz, CDCl3) 7.04 (2H, d, J 6.5, m-CH), 6.94 (1H, t, J 6.5, p-CH), 3.75 (3H, s, OCH3), 2.32 (6H, s, CCH3).
Lead Reference
A. W. Baldwin and R. Robinson, J. Chem. Soc., 1934, 1264-1267.
Keywords
aromatics/arenes, ethers