Mitsunobu coupling of an imide with an alcohol
SyntheticPage 4
DOI:
Submitted: June 7, 2001, published: June 8, 2001
Authors
Graham Sibley (gem.sibley@bris.ac.uk)
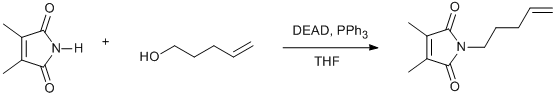
Chemicals
2,3-dimethyl maleimide (1eq)
4-penten-1-ol (1.1eq)
diethylazodicarboxylate (1.5eq)
triphenylphosphine (1.5eq)
THF (0.2M)
4-penten-1-ol (1.1eq)
diethylazodicarboxylate (1.5eq)
triphenylphosphine (1.5eq)
THF (0.2M)
Procedure
To a stirring solution of the maleimide and triphenylphosphine in THF at zero under nitrogen was added the alcohol in THF (10ml) and then DEAD dropwise. The reaction was allowed to warm to room temperature and then left stirring for 2.5 hrs after which time the reaction mixture was concentrated in vacuo. The resultant crude prduct was purified by flash column chromatography.
Author Comments
This is a good method for forming carbon nitrogen bonds. On a large scale to remove the triphenylphosphine oxide produced the crude mixture is cooled down to zero and ether added, followed by trituration. This results in a white solid which can be removed by filteration (wash with cold ether). The mother liquor is concentrated in vacuo and purified as above.
Data
5.68-5.72 (1H, m, CH), 5.06-4.94 (2H, m, CH2), 3.49 (2H, t, CH2 J = 7.3), 1.96 (6H, s, 2 x CH3), 2.20-1.94 (2H, m, CH2), 1.69 (2H, q, CH2, J = 7.3)
Lead Reference
Kevin I. Booker-Milburn, Christopher E. Anson, Cole Clissold, Nicola J. Costin, Richard F. Dainty, Martin Murray, Dhiren Patel, Andrew Sharpe, European Journal of Organic Chemistry, 2001, 1473-1482.
Keywords
alcohols
Comments
No yield?
By istoutgrill on April 27, 2010
Review
Thanks for the reaction. There is also a review on the topic, giving you several similar reactions in 5.1. N-Alkylation Using Phthalimide and Related Imides. See: Mitsunobu and Related Reactions: Advances and Applications, Chem. Rev. 2009, 109, 2551–2651, DOI: 10.1021/cr800278z.
By Marcus Knappert on November 5, 2015