Bromination of an alpha, beta unsaturated ester to give a gamma bromo unsaturated ester
SyntheticPage 39
DOI:
Submitted: August 14, 2001, published: August 15, 2001
Authors
Jonathan Wilden (jonathan_wilden@hotmail.com)
A contribution from
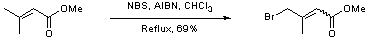
Chemicals
N-bromosuccinimide (recrystallised from water)
AIBN (Acros)
Methyl-3, 3-dimethylacrylate (Aldrich)
Chloroform
AIBN (Acros)
Methyl-3, 3-dimethylacrylate (Aldrich)
Chloroform
Procedure
To a solution of methyl-3, 3-dimethylacrylate (2.0 g, 18 mmol), in chloroform (30 mL), was added N-bromosuccinimide (3.6 g, 20 mmol) and AIBN (50 mg, 0.3 mmol). The mixture was heated to reflux for 3 hours after which time excess NBS and succinimide was removed by filtration. The filtrate was concentrated in vacuo and the resulting red oil purified by colum chromatography (20% ether / petrol) to yield the brominated product as an inseparable mixture of cis and trans isomers (2.4 g, 12.5 mmol, 69%).
Author Comments
This reaction has been repeated a number of times and is pretty reliable on a variety of scales (from mg to g). Sometimes however, carbon tetrachloride proves to be a better choice of solvent due to its higher boiling point. Often the product bromides co-run on tlc with the starting materials.
Data
1H NMR (300 MHz, CDCl3) Trans isomer: 5.90 (1H, s), 3.88 (2H, s), 3.62 (3H, s), 2.21 (3H, s). Cis isomer: 5.73 (1H, s), 3.75 (2H, s), 3.61 (3H, s), 2.00 (3H, s).
Lead Reference
Cameron, D. W.; Gan, C. Y.; Griffiths, P. G.; Pattermann, J. A.; Aust. J. Chem., 1998, 51, 421.