Dehydration of chromium(III) chloride
SyntheticPage 33
DOI:
Submitted: July 16, 2001, published: July 16, 2001
Authors
Peter Scott (peter.scott@warwick.ac.uk)
A contribution from
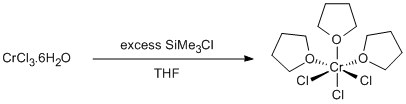
Chemicals
Chromium(III) chloride hexahydrate (Aldrich)
THF (distilled but not necessarily dry)
Trimethylchlorosilane (Aldrich)
Petroleum ether (b.p. 40/60, distilled from Na/K alloy or otherwise thoroughly dry)
THF (distilled but not necessarily dry)
Trimethylchlorosilane (Aldrich)
Petroleum ether (b.p. 40/60, distilled from Na/K alloy or otherwise thoroughly dry)
Procedure
Chromium(III) chloride hexahydrate (2.66 g, 10 mmol) and THF (20 ml) were placed in a three-neck 100 ml round bottomed flask equipped with a water condenser and large teflon stir-bar. The system was flushed with argon. Trimethylchlorosilane (32 ml, 253 mmol) was added dropwise with stirring to the slurry. The colour of the reaction mixture changed from dark green to deep purple. The reaction mixture was heated to reflux overnight (15 h) over which time the solution turned a lighter purple. On standing and cooling a purple solid precipitated from the solution. This was collected on an inert gas frit and washed with 40/60. Alternatively the solution could be decanted off and the solid washed in situ. The solid was dried in vacuo. Yield = 3.14 g, 84 %.
Author Comments
Chris Sanders first performed this prep in my group and developed this improvement. It is based on an Inorganic Syntheses prep which in our hands sometimes gives an incompletely dehydrated product. This may be dependent on the source of the hydrated chloride. In any event, heating overnight gives a good yield of material which most importantly does not give an O-H absorption (3500-3300 cm-1).
Data
IR (Nujol) cm-1: 1617, 1261, 1012, 925, 856, 800, 451.
Lead Reference
Boudjouk, P.; So, J-H. Inorganic Syntheses, Vol. 19, 108-111