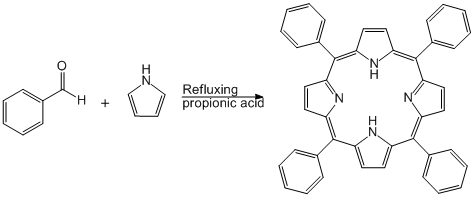
Condensation of Pyrrole with Benzaldehyde Under Adler Conditions
SyntheticPage 316
DOI:
Submitted: October 21, 2010, published: October 21, 2010
Authors
Dan Carney (daniel_carney@brown.edu)
A contribution from
Chemicals
Propionic Acid
Pyrrole
Benzaldehyde
Methanol
Methylene Chloride
Procedure
Pyrrole was freshly distilled. benzaldehyde and propionic acid, purchased recently, were used as is. Pyrrole (1.9mL, 2.69E-2mol) and benzaldehyde (3.0mL, 2.69E-2mol) were combined in a graduated cylinder then agitated until thoroughly mixed. Propionic acid (100mL) was added to a 250 mL round bottom flask fixed with a cold water condenser and heated to reflux. The benzaldehyde and pyrrole mixture was added to the refluxing propionic acid and allowed to react for 30 minutes. Color change to red/brown occurred rapidly and by the end of the reaction the crude solution was opaque. The crude solution was cooled to room temperature then filtered using a Buchner funnel.
The reaction flask and filtered solid were washed with methanol leaving behind shiny purple crystals. The filtered solid as well as the solid remaining in the reaction flask were then washed with methylene chloride into an oven dried and pre-weighed round bottom flask. The solution was concentrated to dryness then weighed for yield determination.
Mass Collected: 716mg
%Yield: 17.3%
Author Comments
The reaction is simple and reliable. The challenge is achieving optimum yield. Reactant concentrations and reaction time are very important. For optimal yield, reactant concentration should be approximately 0.27M. Although the Adler synthesis of TPP constitutes a quick and reliable method for synthesizing tetraarylporphyrins, it is by nature a very low yielding reaction. Inspection of the mechanism for porphyrin formation reveals why.
If available, dry propionic acid gives greater yields. Make sure both pyrrole and benzaldehyde are fresh and in high purity. I recommend storing both in sealed containers under nitrogen and in a refrigerator. Chromatography on the product is not necessary. Chlorin side product can be oxidized to porphyrin by refluxing the reaction product with DDQ in toluene.
Data
HNMR data in chloroform-d:
2H singlet -2.8ppm
12H multiplet 7.7ppm
8H doublet 8.1ppm
8H singlet 8.8ppm
Lead Reference
Adler, A. D.; Longo, F. R.; Finarelli, J. D.; Goldmacher, J.; Assour, J.; Korsakoff, L. J. Org. Chem. 1967, 32, 476-476.
Keywords
Adler, aldehydes, aromatics/arenes, benzaldehyde, condensation, heterocyclic compounds, oxidation, pyrrole
Comments
Microwave Oven Reaction
Some time ago we discovered that heating in a sealed Teflon container in a microwave oven could reduce the reaction time considerably. Yields were never higher than 20% though and the work was never followed up or published.
By Robert Lancashire on November 4, 2010
Reference
This paper reports a typical synthesis of meso-tetraphenylporphyrin with 35% yield.
De Paula, R., Faustino, M. A. F., Pinto, D. C., Neves, M. G. and Cavaleiro, J. A. S., "Kinetic study of meso-tetraphenylporphyrin synthesis under microwave irradiation." Journal of Heterocyclic Chemistry, (2008), 45, 453–459.
doi:10.1002/jhet.5570450224
By Leandro Pedrosa on April 1, 2011
reference
In this paper: "Preparation and characterization of SDS-stabilized hydrophobic porphyrinic nanoaggregates in water". Journal of Porphyrins and Phthalocyanines. 16(3) 267-272, 2012.DOI: 10.1142/s1088424612500320
was reported, a modification of Adler synthesis method and was obtained 78% yield
By Luis Maqueira on June 4, 2013