Nickel chloride catalyzed Biginelli reactions
SyntheticPage 315
DOI:
Submitted: September 18, 2009, published: September 22, 2009
Authors
Sirin Gülten (sgulten@comu.edu.tr)
A contribution from
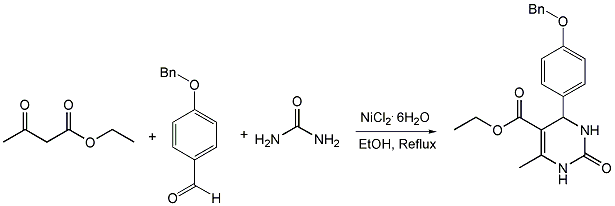
Chemicals
Ethyl acetoacetate (Acros Organics), 4-benzyloxybenzaldehyde (Acros Organics), urea (Merck), nickel chloride hexahydrate (Merck), 37% HCl (Merck).
Procedure
A solution of ethyl acetoacetate (2.5 mmol), 4-benzyloxybenzaldehyde (2.5 mmol), urea (3.75 mmol), nickel chloride hexahydrate (0.62 mmol) and conc. HCl (2 drops) in EtOH (5 mL) were heated under reflux for 7h. After cooling to room temperature the reaction mixture was poured onto crushed ice (20 g) and stirred for 10 min. The crude product was filtered, washed with cold water (2x10 mL) and then recrystallized from ethanol afford white crystals. Yield (0.78g, 85%).
Author Comments
This reaction was carried out with ethyl acetoacetate and thiourea to give the corresponding Biginelli product in good yield. The author thanks Çanakkale Onsekiz Mart University (BAP 2008/28) for financial support.
Data
White crystal, Rf = 0.21 (50% ethyl acetate/hexane), m.p. 190-193 oC, IR (KBr): 3242, 3113, 2977, 1717, 1703, 1647, 1508 cm-1. 1H-NMR (400MHz, DMSO-d6):ppm = 9.15 (br s, 1H, NH), 7.67 (br s, 1H, NH), 7.40-7.32 (m, 5H, Har), 7.15 (d, 2H, J= 8.7 Hz, Har), 6.97 (d, 2H, J= 8.7 Hz, Har), 5.09 (m, 3H, CH2O + CHNH), 3.99 (q, 2H, J= 7.09 Hz, CH3CH2O), 2.24 (s, 3H, CH3), 1.10 (t, 3H, J= 7.07 Hz, CH3CH2O). 13C-NMR (100MHz, DMSO-d6): ppm = 165.84, 157.99, 152.60, 148.50, 137.77, 137.58, 128.88, 128.25, 128.09, 127.88, 115.07, 100.00, 69.64, 59.62, 53.81, 18.23, 14.57.
Lead Reference
J. Lu, Y. Bai, Synthesis 2002, 466-470.
Other References
J. Lu, H. Ma, Synlett 2000, 63-64.