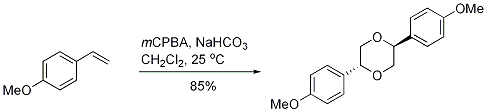
MCPBA mediated cyclodimerization of p-methoxystyrene to 2,5-di(p-methoxyphenyl)-1,4-dioxane
SyntheticPage 313
DOI:
Submitted: July 10, 2009, published: July 14, 2009
Authors
Ishmael Masesane (masesane@mopipi.ub.bw)
A contribution from
Chemicals
p-Methoxystyrene (Aldrich), CH2Cl2 (Aldrich), NaHCO3 (Aldrich), MCPBA (Aldrich)
Procedure
MCPBA (0.62 g, 3.57 mmol) in 20 ml dichloromethane was added slowly over a period of 10 minutes to a stirred and cooled (0oC) mixture of 4-methoxystyrene 98 (0.40 g, 2.97 mmol) and NaHCO3 (1.0 g, 3.6 mmol) in 80 ml dichloromethane. The reaction mixture was stirred at room temperature for 2 hours and then saturated NaHCO3 (50 ml) was added. The organic layer was removed. The aqueous phase was extracted with dichloromethane (2 x 10 ml) and the organic fractions were combined, dried (MgSO4) and concentrated under reduced pressure. The residue was subjected to flash chromatography eluting with petroleum ether/ethyl acetate (PE; 5:1) to give a yellow jelly (0.34 g, 85 %).
Author Comments
The reaction is thought to proceed through epoxidation of p-methoxystyrene then cyclodimerization of the epoxide. The reaction was performed under atmospheric air and the reagents as used without further purification.
Data
Proton NMR (300 MHz, CDCl3): 3.71 (6H, s, 2 x MeO), 3.81 (2H, dd, J = 12.1 Hz and 3.9 Hz, H-3a, 6a), 3.94 (2H, dd, J = 12.1 Hz and 7.8 Hz, H-3b, 6b), 6.05 (2H, q, J = 7.8 Hz and 3.9 Hz, H-2, 5), 6.83 (4H, d, J = 8.7 Hz, H-2’, 6’, 2’’, 6’’), 7.33 (4H, d, J = 8.7 Hz, H-3’, 5’, 3’’, 5’’). C-13 NMR (75 MHz, CDCl3): 55.2 (MeO), 65.5 (C-3, 6), 77.6 (C-2, 6), 114.1 (C-2’, 6’, 2’’, 6’’), 128.2 (3’, 5’, 3’’, 5’’), 129.1 (1’, 1’’), 159.7 (4’, 4’’).
Lead Reference
Concellon, J. M.; Bernad, P. L.; de Solar, V.; Garcia-Granda, S.; Diaz, M. R. Adv. Synth. Catal. 2008, 350, 477 doi:10.1002/adsc.200700486
Other References
N/A
Keywords
Comments
It's not clear what MCPBA is. It might be worth defining what mCPBA is for the novice reader
By Antony Williams on November 9, 2009