Acid mediated cyclodimerization of styrene oxide to 2-benzyl-4-phenyl-1,3-dioxalane
SyntheticPage 312
DOI:
Submitted: July 10, 2009, published: July 14, 2009
Authors
Ishmael Masesane (masesane@mopipi.ub.bw)
A contribution from
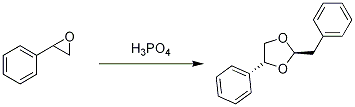
Chemicals
Styrene (Aldrich), MCPBA (Aldrich) Phosphoric acid (Aldrich)
Procedure
Phosphoric acid (0.2 ml) was added drop-wise to styrene epoxide (0.5 g, 4.17 mmol) and the mixture was stirred at room temperature for 120 minutes. Saturated sodium bicarbonate (20 ml) was added and the mixture was extracted with dichloromethane (2 x 15 ml) and the organic fractions were combined, dried (magnesium sulphate) and concentrated under reduced pressure. The residue was subjected to flash chromatography eluting with petroleum ether/ethyl acetate (4:1) to give the product as a clear jelly (0.31 g, 61 %).
Author Comments
All reactions were performed under atmospheric pressure and all reagents were purchased from commercial sources and were used without further purification. The reaction is thought to proceed through a 1,2-hydride shift.
Data
1H NMR (300 MHz, CDCl3): 3.24 (2H, t, J = 5.7, PhCH2), 3.75 (1H, dd, J = 7.8 Hz and 6.3 Hz, H-5), 4.22 (1H, dd, J = 7.8 Hz and 0.9 Hz, H-5), 5.06 (1H, t, J = 6.3 Hz and 0.9 Hz, H-4), 5.38 (1H, t, J = 5.7 Hz, H-2), 7.39-7.44 (10 H, m, Aromatic protons); 13C NMR (75 MHz, CDCl3): 40.8 (PhCH2), 72.0 (C-5), 78.5 (C-4), 105.4 (C-2), 126.4 (C-2'' and C-6''), 126.7 (C-4''), 128.1 (C-1'), 128.3 (C-3'' and C-5''), 128.5 (C-2' and C-6'), 130.0 (C-3' and C-5'), 136.0 (C-1'), 139.4 (C-1'').
Lead Reference
Concellon, J. M.; Bernad, P. L.; de Solar, V.; Garcia-Granda, S.; Diaz, M. R. Adv. Synth. Catal. 2008, 350, 477 doi:10.1002/adsc.200700486
Other References
N/A