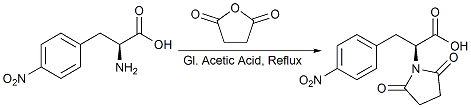
N-Protection of L-Phenyl Alanine using succinic anhydride
SyntheticPage 305
DOI:
Submitted: February 13, 2009, published: February 16, 2009
Authors
Abir Kumar Pal (abirchem2@yahoo.com)
A contribution from
Chemicals
p-Nitro Phenyl Alanine (In House), Succinic Anhydride (CDH); Acetic Acid, Methylene Chloride (Rankem), Iso Propyl Alcohol (Rankem)
Procedure
p-Nitro phenyl alanine (20g, 0.095mol) was placed in a 500 ml 4-neck rbf then charged with acetic acid (200ml). Succinic anhydride (10.4g, 0.104mol) was added and then heated to 100110˚C for 6-8h with continuous stirring. The reaction was monitored by TLC (solvent system: 40% methanol/DCM and 12 drops aqueous ammonia). The reaction mixture was cooled to RT, poured slowly onto ice-cold water (500ml) and then poured onto methylene chloride (100ml)in a separating funnel. The organic layer was extracted with DCM and the solvent removed in vacuo. Isopropyl alcohol (50ml) was added to the resultant gum and stirred for 2-3hrs after which the red colored solid product was observed. The product was filtered through a sinter funnel and washed with IPA (2 x 10ml) and dried under vacuum for 4hrs. Yield: 15g (54%).
Author Comments
The reaction was carried out successfully on scales of up to 1kg.
Data
Rf 0.2 (40% MeOH /DCM). 1HNMR (300 MHz) in DMSO- d6: 2.55 (dd, 4H), 2.66 (d, 2H), 4.92 (t, 1H), 7.41 (d, 2H), 8.09 (d, 2H), 12.57(Broad, COOH). MS (ES+) m/z 293.22 and (ES-) m/z 290.97
Lead Reference
The Practice of Peptide Synthesis, 2nd edition, M. Bodanszky, A. Bodanszky, 1994, page 17, Springer-Verlag Publications.