Alkylation of D-Aspartic acid
SyntheticPage 297
DOI:
Submitted: August 28, 2008, published: August 28, 2008
Authors
Neetisha Mistry (n.s.mistry@warwick.ac.uk)
A contribution from
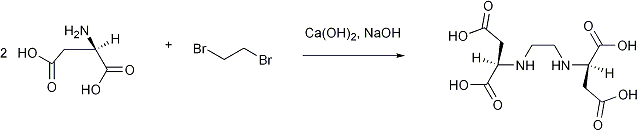
Chemicals
D-Aspartic acid (Aldrich);
Ca(OH)2 ;
NaOH (50% aqueous w/v);
1,2-dibromoethane
Ca(OH)2 ;
NaOH (50% aqueous w/v);
1,2-dibromoethane
Procedure
D-aspartic acid (6.083 g, 0.045 mol), Ca(OH)2 (1.653 g, 0.023 mol), NaOH (50% aqueous w/v, 3.60 g), and deionized water (8.5 ml) were placed in a 3-necked round bottom flask, and fitted with a water condensor. 1,2-dibromoethane (1.6 ml, 3.47 g, 0.018 mol) was added through one of the side arms. The reaction mixture was heated to reflux. Over approximately 6 hours NaOH (50% aqueous w/v, 2.98 g) was added drop wise using a syringe pump, whilst under reflux. Water (20 ml) was added, and the system refluxed for a further hour. The system was cooled to room temperature, then acidified to pH 3 using HCl (35%). A white precipitate was collected and was placed in water (23 ml). NaOH (50% aqueous w/v) was added until pH 11, then HCl (35%) was added carefully until pH 3.5. The white precipitate was collected, and dried under vacuum at 65 °C. Yield: 1.417 g, 26%
Author Comments
The aspartic acid is used in excess to the dibromoethane, there should be a 2:1 stoichiometry.During the addition of NaOH over 6 hours the pH of the reaction mixture is best between pH 9-13. The crude product will include many side products and the unreacted aspartic acid. Since aspartic acid is more soluble in water, it should remain in solution during the recrystallisation.
Data
H-NMR (300MHz, 0K, D2O/NaOH) 2.24 (dd, 2H, 3JHH=8.0Hz, 2JHH=15.1Hz, CH2COO), 2.41 (dd, 2H, 3JHH=5.7Hz, 2JHH=15.1Hz, CH2COO), 2.53 (m, 2H, CH2CH2), 3.29 (dd, 2H, 3JHH=5.7Hz, 3JHH=8.0Hz), 4.79 (solvent peak D2O/NaOH);C-NMR (D2O/NaOH): 41.3 (CH2-CH2 or CH2COO), 46.6 (CH2-CH2 or CH2COO), 61.3 (CH), 179.8 (COO quaternary), 181.5 (COO quaternary); ESI Positive m/z 293.09 ([EDDS + H]+)
Lead Reference
A.Damiamoglou et al. A New Reference Material for UV-visible Circular Dichroism Spectroscopy, Chirality, 2008
Keywords
aspartic acid, dibromoethane, nucleophilic, substitution, amino acids
Comments
I was wondering why do you need Calcium hydroxide? ...Is it for masking carboxyl group?
By marto lesh on August 31, 2008
The calcium hydroxide is another method to raise the pH of the reaction mixture to a more preferable range of 9-13, aswell as the sodium hydroxide.
By Neetisha Mistry on September 6, 2008
I suspect that the Ca2+ ion preorganises the reagents. The product EDDS ligand is a known chelator for Ca2+, and this enantiomer is biodegradable unlike EDTA.
By Peter Scott on September 6, 2008