Reduction of chroman-2-ones to lactols using diisobutylaluminium hydride
SyntheticPage 29
DOI:
Submitted: July 11, 2001, published: July 11, 2001
Authors
Jonathan Wilden (jonathan_wilden@hotmail.com)
Chemicals
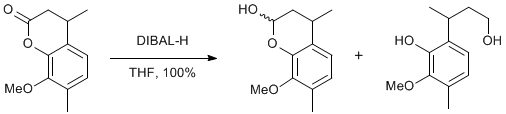
8-Methoxy-4,7-dimethylchroman-2-one
THF (distilled sodium/benzophenone)
Diisobutylaluminium hydride (1.0 M in Hexane, Acros)
Magnesium sulfate (BDH)
THF (distilled sodium/benzophenone)
Diisobutylaluminium hydride (1.0 M in Hexane, Acros)
Magnesium sulfate (BDH)
Procedure
To a precooled (-78oC) solution of 8-Methoxy-4, 7-dimethylchroman-2-one (4.0 g, 19 mmol) in THF (150 mL) was added diisobutylaluminium hydride (23 mL, 23 mmol) dropwise over 10 minutes and the resulting clear solution stirred at -78oC for 3h. Water (5 mL) was added dropwise and the solution allowed to warm to RT. Magnesium sulfate (ca. 15 g) was added and the suspension was filtered and the filtrate concentrated in vacuo. The crude residue was purified by flash column chromatography (20-50% ether/petroleum ether 40-60oC) to yield firstly 8-methoxy-4, 7-dimethylchroman-2-ol as a viscous clear oil (3.2 g, 15 mmol, 82%) then 3-(2-hydroxy-3-methoxy-4-methylphenyl)-butan-1-ol as a white solid (0.6 g, 4.0 mmol, 18%). Product lactol is a 3 : 1 mixture of diastereoisomers.
Author Comments
Addition of the DIBAL-H must be carried out slowly and care should be taken to avoid temperature rise above -70oC to minimise further reduction to the diol. Also the lactol product co elutes with the chromenone starting material, therefore it is best to leave the reaction for at least 3 hours to ensure complete conversion.
Data
Major isomer: (1H NMR, CDCl3) 6.83 (1H, ArH), 6.72 (1H, ArH), 5.72 (1H, OCHO), 4.09 (1H, OH), 3.71 (s, OCH3), 3.15 (1H, ArCH), 2.25 (3H, ArCH3), 2.14-2.00 (1H, CH2), 1.79-1.64 (1H, CH2), 1.24 (3H, CH3). Minor isomer: 6.78 (1H, ArH), 6.72 (1H, ArH), 5.57 (1H, OCHO), 4.19 (1H, OH), 3.73 (s, OCH3), 2.94 (1H, CH), 2.25(3H, ArCH3), 2.14-2.00 (1H, CH2), 1.79-1.64 (1H, CH2), 1.29 (3H, CH3).
Lead Reference
D.C. Harrowven, J.D. Wilden, M.J. Tyte, M.B. Hursthouse, S.J. Coles, Tetrahedron Letters, 2001, 42, 1193
Keywords
DIBAL, diisobutylaluminium hydride, elimination, lactol, lactone, Reduction