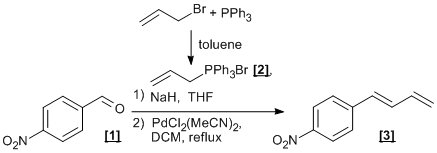
Wittig reaction of aldehydes and subsequent Pd-catalysed diene isomerisation
SyntheticPage 285
DOI:
Submitted: June 10, 2008, published: June 10, 2008
Authors
Maria Paz Munoz-Herranz (paz.munoz@iqog.csic.es)
A contribution from
Chemicals
p-Nitro-benzaldehyde [1] (Aldrich).
Triphenylphosphine (Aldrich).
Allyl bromide (Aldrich).
Tetrahydrofuran (dry, inhibitor-free, obtained by passage through an Anhydrous Engineering drying column).
Toluene (dry, inhibitor-free, obtained by passage through an Anhydrous Engineering drying column).
Sodium hydride (60% in oil) (Aldrich).
PdCl2(MeCN)2 (made from PdCl2 in hot MeCN).
Dichloromethane (dry, inhibitor-free, obtained by passage through an Anhydrous Engineering drying column).
Solvents for extraction and chromatography were technical grade.
Triphenylphosphine (Aldrich).
Allyl bromide (Aldrich).
Tetrahydrofuran (dry, inhibitor-free, obtained by passage through an Anhydrous Engineering drying column).
Toluene (dry, inhibitor-free, obtained by passage through an Anhydrous Engineering drying column).
Sodium hydride (60% in oil) (Aldrich).
PdCl2(MeCN)2 (made from PdCl2 in hot MeCN).
Dichloromethane (dry, inhibitor-free, obtained by passage through an Anhydrous Engineering drying column).
Solvents for extraction and chromatography were technical grade.
Procedure
i) Formation of Allyltriphenylphosphonium bromide [2].
To a solution of triphenylphosphine (30.31 g, 115 mmol) in toluene (50 mL), was added allyl bromide (10 mL, 115 mmol) and the mixture stirred at room temperature. After 18 h, the white precipitate formed was filtered, washed with cold toluene and petroleum ether and dried under vacuum to give allyltriphenylphosphonium bromide [2] as a white solid (40.58 g, 106 mmol, 92 %).
ii) Formation of cis/trans-p-nitro-1-phenylbuta-1,3-diene E/Z-[3] (1:1).
Under an inert atmosphere, allyltriphenylphosphonium bromide [2] (2.54 g, 6.62 mmol) and sodium hydride (60% in oil, 317 mg, 7.94 mmol) in dry THF (15 mL) were cooled to 0oC with vigorous stirring. After 3 h, p-nitro-benzaldehyde [1] (1.00 g, 6.62 mmol) was added with vigorous stirring. Once the addition of [1] was complete, the reaction was allowed to warm to room temperature. Once all starting material was consumed (about 16h) the reaction was concentrated. Then, pentane was added and the mixture filtered through a pad of silica (washing with Hexane) to give cis/trans-p-nitro-1-phenylbuta-1,3-diene E/Z-[3] (1:1) as an orange solid (276 mg, 1.57 mmol, 24 %).
iii) E -p-nitro-1-phenylbuta-1,3-diene.
The E/Z (1:1) mixture of p-nitro-1-phenylbuta-1,3-diene (105 mg, 0.60 mmol) was dissolved in DCM (6 mL) under inert atmosphere. PdCl2(MeCN)2 (5 mol%, 7.8 mg, 0.03 mmol) was added and the mixture heated at reflux for 24 h. After cooling at room temperature, the solvent was concentrated, filtered through a pad of silica and washed with Hexane to give the product as a pale yellow solid (88 mg, 0.50 mmol, 84%).
Author Comments
The same procedure has been applied for the synthesis of trans-p-trifluoromethyl-1-phenylbuta-1,3-diene, using NaHMDS (1M in THF) as base for the Wittig reaction with similar results. The Pd-catalysed isomerisation in this case took 2 days under reflux to give an overall yield of 31%.
The Wittig reaction using allylphosphonium bromide gives a mixture of E/Z diene due to an isomerisation of the allyl phosphonium ylide after deprotonation with the base. Use of different bases or temperatures gave the same 1:1 mixture.
Manipulations involving air-sensitive materials were carried out on a vacuum line under nitrogen, employing standard Schlenk techniques.
This work has been carried out in collaboration with Jennifer L. Slaughter from the Booker-Milburn group at University of Bristol.
Data
1H NMR (CDCl3, 300 MHz): 5.35 (d, 1H, 3JHH = 9.7 Hz, C[4]Htrans); 5.50 (d, 1H, 3JHH = 17.0 Hz, C[4]Hcis); 6.55 (dt, 1H, 3JHH = 17.0 Hz, 10.6 Hz, C[3]H); 6.54 (d, 1H, 3JHH = 15.7 Hz, C[1]H); 6.93 (dd, 1H, 3JHH = 15.7 Hz, 10.5 Hz, C[2]H), 7.52 (app. d, 2H, JHH = 8.8 Hz, CHo-arom); 8.18 (app. d, 2H, JHH = 8.8 Hz, CHm-arom).
13C NMR (CDCl3, 300 MHz): 146.8, 143.7, 136.4, 134.0, 130.4, 126.8, 124.1, 121.0.
Lead Reference
i) Wittig reaction with allyl phosphonium salts: M. Ackermann, S. Berger, Tetrahedron, 2005, 61, 6764-6771.
ii) Pd-Catalysed isomerisation: G. C. Lloyd-Jones, E. Tan, M. P. Muñoz, unpublished results.