Wittig Reaction of cinnamaldehyde
SyntheticPage 284
DOI:
Submitted: June 10, 2008, published: June 10, 2008
Authors
Jennifer Louise Slaughter (chjlk@bris.ac.uk)
A contribution from
Chemicals
Trans-Cinnamaldehyde [1] (Aldrich, distilled before use).
Triphenylphosphine (Aldrich).
Methyl iodide (Aldrich).
Tetrahydrofuran (dry, inhibitor-free, obtained by passage through an Anhydrous Engineering drying column).
Sodium hexamethyldisilazane (Aldrich, 1.6M in THF).
Solvents for extraction and chromatography were technical grade.
Triphenylphosphine (Aldrich).
Methyl iodide (Aldrich).
Tetrahydrofuran (dry, inhibitor-free, obtained by passage through an Anhydrous Engineering drying column).
Sodium hexamethyldisilazane (Aldrich, 1.6M in THF).
Solvents for extraction and chromatography were technical grade.
Procedure
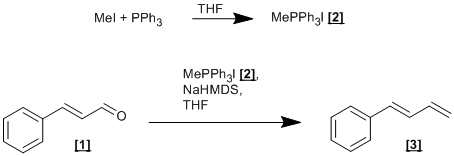
(a) Formation of Methyltriphenylphosphonium iodide [2].
To a solution of triphenylphosphine (26.2g, 100 mmol) in THF (100 mL), was added methyl iodide (6.23 mL, 100 mmol). After approximately three hours, a white precipitate formed which was filtered, washed with cold THF and dried in air to give methyltriphenylphosphonium iodide [2] as a fine white crystalline solid (30.1 g, 84.3 mmol, 84 %).
(b) Formation of trans-1-Phenylbuta-1,3-diene [3].
Under an inert atmosphere, a solution of methyltriphenylphosphonium iodide [2] (12.9 g, 36 mmol) in dry THF (50 mL) was cooled to 0oC. Sodium hexamethyldisilazane (1.6 M in THF, 22.5 mL, 36 mmol) was added dropwise with vigorous stirring. After approximately twenty minutes a clear solution was formed to which trans-cinnamaldehyde [1] (3.78 mL, 30 mmol) was added dropwise with vigourous stirring. Once the addition of [1] was complete, the reaction was allowed to warm to room temperature. Once all starting material was consumed (as observed by TLC; eluting with 1 % Et2O in pentane, on silica) the reaction was filtered through a pad of silica and concentrated ‘in vacuo’. Purification by column chromatography (eluting with 1 % Et2O in pentane, on silica) and Kugelrohr distillation (70oC at 5 mm/Hg) gave trans-1-phenylbut-1,4-diene [3] as a colourless oil (3.47 g, 26.7 mmol, 89 %).
Author Comments
Acknowledgement and thanks are made to Dr. M. Paz Munoz, University of Bristol, for essential input and support.
The methyltriphenylphosphonium salt [2] can be formed with either methyliodide or methylbromide; the counterion having no effect on the yield of the Wittig reaction.
Due to the volatile nature of the trans-phenylbutadiene product, it is important to avoid high vacuum or high temperature when concentrating solutions.
Manipulations involving air-sensitive materials were carried out on a vacuum line under nitrogen, employing standard Schlenk techniques.
The above procedure can be applied to other commercially available trans-cinnamaldehyde derivatives; for example, the p-methoxy-trans-cinnamaldehyde was synthesised via the above method to give the trans-1-(p-methoxy)phenylbuta-1,3-diene in 98 % yield.
Data
1H NMR (CDCl3, 300 MHz): 5.17 (d, 1H, 3JHH = 10.0 Hz, C[4]Htrans); 5.33 (d, 1H, 3JHH = 16.8 Hz, C[4]Hcis); 6.49 (dt, 1H, 3JHH = 10.2 Hz, 16.8 Hz, C[3]H); 6.57 (d, 1H, 3JHH = 15.8 Hz, C[1]H); 6.79 (dd, 1H, 3JHH = 16.8 Hz, 10.0 Hz, C[2]H), 7.24 (m, 2H, CHo-arom); 7.33 (m, 2H, CHm-arom); 7.41 (m, 1H, CHp-arom).
13C NMR (CDCl3, 300 MHz):117.6 (C[4]H2); 126.4 (CHm-arom); 127.6 (CHp-arom); 128.6 (CHo-arom); 129.6 (C[2]H); 132.8 (C[1]H); 137.0 (Ci-arom); 137.2 (C[3]H).
Lead Reference
H. Lebel, V. Paquet, J. Am. Chem. Soc., 2004, 126, 320 – 328.
Other References
i) H. Lebel, V. Paquet, C. Proulx, Angew. Chem. Int. Ed., 2001, 40, 2887 – 2890. ii) K. Kobayashi, S. Oka, T. Okamoto, S. Tanimoto, J. Org. Chem., 1988, 53, 4897 – 4901.