TTF III. Decarboxylation of 1,3-dithiole-2-thione-4,5-dicarboxylic acid
SyntheticPage 279
DOI:
Submitted: June 5, 2008, published: June 5, 2008
Authors
Nikola Paul Chmel (n.chmel@warwick.ac.uk)
A contribution from
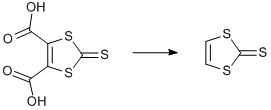
Chemicals
Procedure
1,3-Dithiole-2-thione-4,5-dicarboxylic acid (15.2 g, 0.067 mol) was refluxed in pyridine (70 ml) for 3 h, then mixture was left stirred overnight. The mixture was concentrated under reduced pressure with gentle heating to thick black oil (yellow film starts showing on the neck of the flask). Resulting oil was refluxed with hexane (200 ml) for 0.5 h at 95°C. Hot hexane was decanted and cooled in acetone/dry ice bath. Yellow precipitate was collected by filtration without washing, dried in vacuo. Extraction procedure was repeated at least four times. Overall yield 7.4g (82%)
Author Comments
Has been performed several times with similar yields. Product may be stored for extended periods of time in dark, tightly closed bottle.
Data
1H NMR (400MHz, Acetone-d6, 298K) 7.52 (s, CH); 13C NMR (400MHz, Acetone-d6, 298K) 132.28 (very weak), 207.11
Lead Reference
L.R. Melby, H.D. Hartzler, W.A. Sheppard, J.Org. Chem. Vol. 39, No. 16, (1974) 2456-2458
Keywords
carboxylic acids, decarboxylation, heterocyclic compounds