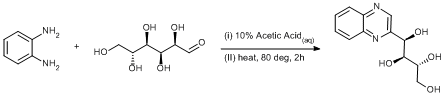
Condensation of diamine with glucose
SyntheticPage 269
DOI:
Submitted: June 1, 2008, published: June 1, 2008
Authors
Jan Martin Becker (J.M.Becker@warwick.ac.uk)
A contribution from
Chemicals
ortho-phenylenediamine (Aldrich);
D-glucose (Aldrich);
Acetic acid (Fisher);
D-glucose (Aldrich);
Acetic acid (Fisher);
Procedure
Synthesis of (1R,2S,3R)-1-(quinoxalin-2-yl)butane-1,2,3,4-tetraol: (30.54 g, 282 mmol) ortho-phenylenediamine was placed in a 500 ml round-bottom flask and suspended in 10% acetic acid (140 ml); D-glucose (10.16 g, 282 mmol) was added in five portions over 30 min. and the solution changed to a deep golden colour. The mixture was heated to 80ºC for 2 h and changed colour to a deep red, left to cool to ambient temperature and stored at +4ºC for 12 h. A mass of golden plate like crystals are deposited and the isolated by filtration, the solid was washed with a little cold water (2 x 20 ml) and dried in an vacuum oven (60ºC, 0.02 mm Hg) for 5 h. (25.0 g, 35 %).
Author Comments
The methodology can easily be adapted for other sugars such as fructose etc. The quoted yield is close to reported literature results. Has been performed several times.
Data
1H NMR (300 MHz, DMSO-d6): 9.19 (s, 1H, ArH), 8.05-8.11 (m, 2H, ArH), 5.63 (d, 2H), 5.17 (d, 1H), 4.74 (d, 1H), 4.64 (d, 1H), 4.42 (t, 1H), 3.65-3.72 (m, 3H). 13C NMR (75 MHz, DMSO-d6): 159.4, 145.2, 141.0, 140.8, 129.9, 129.2, 128.8, 128.6, 74.3, 72.5, 71.2, 63.5.
Lead Reference
A. E. Harms, Org. Process Res. Dev., 2004, 8, 666-669.
Keywords
amines, aromatics/arenes, carbohydrates and sugars, condensation