Williamson etherification using an aminoalcohol
SyntheticPage 268
DOI:
Submitted: May 30, 2008, published: May 30, 2008
Authors
Suzanne Elizabeth Howson (s.e.howson@warwick.ac.uk)
A contribution from
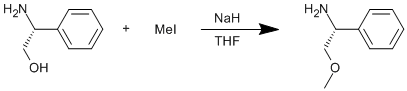
Chemicals
Phenyl Glycinol (Made according to Literature Prep for (S)-tert-Leucinol (Evans, D. A.; Peterson, G. S.; Johnson, J. S.; Barnes, D. M.; Campos, K. R.; Woerpel, K. A., J. Org. Chem., 1998, 63, 4541-4544.)),
Iodomethane (Aldrich),
THF (distilled from potassium),
NaH (Aldrich, washed with dry ether then dry pentane).
Iodomethane (Aldrich),
THF (distilled from potassium),
NaH (Aldrich, washed with dry ether then dry pentane).
Procedure
D-(-)-a-Phenylglycinol (2.00 g, 14.6 mmol) was dissolved in dry THF (20 ml) and was added dropwise to a stirred suspension of sodium hydride (0.72 g, 30.0 mmol, 2.05 eq) in dry THF (10 ml). The solution was stirred for 1 h at ambient temperature. Iodomethane (0.96 ml, 15.4 mmol) was added dropwise and the solution was stirred for 1 h at ambient temperature. At this point, the solution was heated to reflux (65oC) for a further 2 h before cooling to ambient temperature, followed by the addition of brine (40 ml). The product was extracted with diethyl ether (4 × 60 ml), dried over sodium sulphate and the solvent was removed to leave a clear oil (crude yield = 1.80 g). This crude product was purified by Kugelrohr distillation to give a clear oil (bp 70oC under high vacuum). Purified yield = 1.38 g, 9.1 mmol, 63%.
Author Comments
Other ethers, such as the benzyl ether, have been made by the same method.
Data
1H NMR (300 MHz, 298 K, CDCl3) 7.33-7.16 (5H, m, Ph), 4.12 (1H, dd, 3JHH = 4 Hz, 9 Hz, CH), 3.43 (1H, 2JHH = 5 Hz , 3JHH = 4 Hz, CH2), 3.32-3.26 (4H, m, CH2, CH3), 1.67 (2H, s, NH2).
13C{1H} NMR (75 MHz, 298 K, CDCl3) 142.56, 128.44, 127.40, 126.79, 79.01, 58.93, 55.42.
Lead Reference
Bream, R. N.; Ley, S. V.; McDermott, B.; Procopiou, P. A. Journal of the Chemical Society-Perkin Transactions 1 2002, 2237-2242.
Other References
Evans, D. A.; Peterson, G. S.; Johnson, J. S.; Barnes, D. M.; Campos, K. R.; Woerpel, K. A., J. Org. Chem., 1998, 63, 4541-4544.