Cerium(III) Chloride Mediated Addition of Grignard Reagents to chloro-Azaphthalide
SyntheticPage 266
DOI:
Submitted: April 19, 2008, published: April 22, 2008
Authors
Biswajit Panda (biswajitchem@yahoo.co.in)
A contribution from
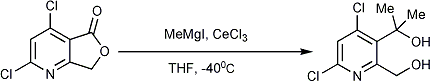
Chemicals
Calcium chloride, cerium(III) chloride hepta hydrate, Methyl magnesium iodide(3M solution in ether, Aldrich, as received),
Tetrahydrofuran, Diethyl ether, Sodium bicarbonate, Sodium chloride, Magnesium sulfate.
Tetrahydrofuran, Diethyl ether, Sodium bicarbonate, Sodium chloride, Magnesium sulfate.
Procedure
CeCl3.7H2O (6.56 g, 17.64 mmol) was taken in a 50 mL two neck round bottom flask, one neck was closed by glass stopper and another neck was connected with a guard tube (filled with anhydrous CaCl2) through a vacuum adaptor. The opposite end of the guard tube was connected with a vacuum pump. Then the RB flask was heated to 120°C in vacuum for 12 h, then cooled to room temperature and the vacuum in inside the RB was released as well as fitted under N2. Then magnet was added through by another neck by quickly opening of glass stopper and it was then quickly replaced by a septum.After that, 20 mL of dry THF was added by a syringe and the resulting slurry was stirred vigorously for 1 hour at room temperature. A solution of Azaphthalide, 2,4-Dichloro-7H-furo[3,4-b]pyridin-5-one (500 mg, 2.45 mmol) in 5 mL dry THF was added via syringe (rinsed with 1 mL dry THF) and the mixture was stirred for 30 min at room temperature before it was cooled to -40°C. To the chilled solution was added 5.7 mL of MeMgI (3.0 M solution in Et2O, 17.15 mmol) and stirring was continued for 30 min at -40°C, then the mixture was allowed to warm to room temperature and stirring was continued for 3h at same temperature. The reaction mixture was poured into ice and diluted with Et2O (60 mL). The aqueous layer was partitioned several times with Et2O (100 mL total), and the combined Et2O extracts were washed twice with saturated aqueous NaHCO3, once with brine, and dried over Na2SO4. Filtration was followed by removal of solvent under reduced pressure, and the crude residue was purified by silica gel chromatography (gradient elution, 30% EtOAc/Hexanes) to give the title compound 515 mg (89% yield) as a dense oil.
Author Comments
We have tried this reaction six times with methyl magnesium iodide at room temp, over night reflux ,etc but the result is only lactol. After addition of one equivalent of MeMgI, next nucleophilic attuck was very difficult due to steric effect of chlorine. but that problem was successfully overcome by using cerium(III)chloride mediated reaction. This is may be due to more nucleophilicity of organocerium reagents. It is a simple and very useful method for nucleophilic grignard addition in sterically hindered substrate.
Data
1H NMR (200 Mz,CDCl3) 7.27(1H,s,CH), 5.01(2H,s,CH2),3.91(2H,br s, two OH),1.77(6H,s,Two CH3).
Lead Reference
S.E. Reisman, J.M. Ready, A.Hasuoka, C.J. Smith,J.L. Wood, J. Am. Chem. Soc.,2006, 128, 1448 -1449,
Other References
G.Bartoli,E.Marcantoni,M.Petrini, J.Chem. Soc., Chem.Comm. 1993, 18, 1373