Iron-mediated reduction of aromatic nitro- groups to amines.
SyntheticPage 261
DOI:
Submitted: March 22, 2007, published: March 23, 2007
Authors
Andrew McCarroll (andrew.mccarroll@nottingham.ac.uk)
A contribution from
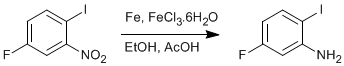
Chemicals
Iron powder (Aldrich, as received)
Iron(III) chloride hexahydrate (Aldrich, as received)
Ethanol (Fisher, absolute, as received)
Acetic acid (Fisher, as received)
5-Fluoro-2-iodonitrobenzene (prepared from 4-fluoro-2-nitroaniline via a Sandmeyer reaction)
Iron(III) chloride hexahydrate (Aldrich, as received)
Ethanol (Fisher, absolute, as received)
Acetic acid (Fisher, as received)
5-Fluoro-2-iodonitrobenzene (prepared from 4-fluoro-2-nitroaniline via a Sandmeyer reaction)
Procedure
5-Fluoro-2-iodonitrobenzene (17.4 g; 65.2 mmol) was dissolved in a mixture of ethanol (100 mL) and acetic acid (12 mL) in a large RB flask (at least 500 mL) equipped with an efficient condenser, and the stirred mixture brought to a gentle reflux. Iron powder (26.6 g) was added, followed immediately by iron(III) chloride hexahydrate (3 g). The mixture was refluxed for a further 3 hours, then cooled and filtered using a Buchner funnel (with the help, when necessary, of the addition of ether or ethanol.)
To the filtrate was added ether (300 mL) and water (300 mL), and the aqueous layer was repeatedly extracted with ether. The combined organic layers were dried and concentrated, giving 5-fluoro-2-iodoaniline and a pure white solid (14.27 g; 92%).
Author Comments
An efficient stirrer bar is perfectly fine to use, although of course the iron sticks to it.
It is necessary to use use an oversized flask because of the vigourous reflux that occurs on addition of the iron.
Iron chloride comes in big yellow blocks. I scraped the blocks using a spatula to get a powder, but this should be done immediately before addition, as it seems to be pretty hygroscopic.
The reaction is clean as far as the product goes, but not as far as waste goes. It is black/brown and messy. Flasks are cleaned with hydrochloric acid, iron residues are treated with HCl, and the combined aqueous wastes bottled for suitable disposal.
The filtration is performed using a Buchner funnel because it is much easier, and sintered funnels block very quickly.
The reaction was always extremely high yielding on this substrate. The product is commercially available, but expensive (from Lancaster, now Alfa).
The method has also been used on more complicated compounds, on a smaller scale (1-2 g SM) in lower yields (50-60%). Again though, the product was pure and didn't require even to be dried prior to concentration - often the product crystallized in the ethanol once the ether had been removed.
Many other methods for this transformation are also available. Tin methods are unpleasant and toxic, palladium methods are likely to remove the iodine. I didn't try zinc, although I did find that indium performed the transformation in reasonable yield - too expensive to be useful on this scale though.
It is worth noting that the iodine functionality remains unchanged in the reaction.
Data
1H nmr. CDCl3. 4.21 (2H, bs, NH2), 6.28 (1H, td, J = 8.5, 2.8), 6.49 (1H, dd, J = 10.5, 2.8), 7.57 (1H, dd, 8.7, 6.2)
Lead Reference
B. Gabriele, G. Salerno, L. Veltri, M. Costa, C. Massera, Eur. J. Org. Chem. 2001, 4607.