Preparation of 1,3,5,7-tetramethyl-2,4,8-trioxa-6-phospha-adamantane
SyntheticPage 257
DOI:
Submitted: January 29, 2007, published: January 31, 2007
Authors
Geoffrey Roff (gjr8@st-andrews.ac.uk)
A contribution from
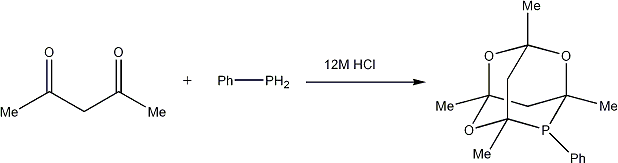
Chemicals
phenylphosphine (Aldrich),
2,4-pentanedione (Lancaster),
hydrochloric acid (37%, Fisher)
2,4-pentanedione (Lancaster),
hydrochloric acid (37%, Fisher)
Procedure
To HCl (37%, N2 saturated, 70ml) in a large Schlenk flask was added 2,4-pentanedione (18.2ml, 0.96mol) in one portion. Nitrogen was bubbled through the mixture for five minutes and the mixture was stirred at room temperature for 1 hour under a nitrogen atmosphere before cooling with an ice bath. Phenylphosphine (7ml, 0.064mmol) was added slowly via a syringe (Care: Phenylphosphine is spontaneously flammable in air and has incredibly pungent odour). The mixture was stirred at room temperature for 4 days. Water (70ml) was added and the white precipitate collected and washed with water (100ml) before drying under vacuum. 13g (70%) of a white solid was collected.
Author Comments
No care needs to be taken in terms of storage of the caged phosphine as it is both air and moisture stable. The phosphine itself has shown unprecedented regioselectivity and activity in the hydroformylation of unsaturated esters.
It is important to perform the reaction in an efficient fumehood as phenylphosphine has a very pungent odour. It is important also to obey good schlenk techniques as phenylphosphine will ignite in air. Phenylphosphine is generally obtained in glass ampules that should be cracked open under a funnel of argon and transferred to a clean dry Young’s flask via syringe and stored under argon out of direct sunlight. Any glassware and other items that come into contact with the phosphine should be thoroughly rinsed with iodine/methanol solution until decolourisation ceases.
Data
1H NMR (300MHz, CDCl3) 1.22 (3H, d J=13.1Hz, CH3), 1.40 (6H,s, 2CH3), 1.73-2.15 (4H, m, 2CH2), 7.30-7.40 (3H, m, 3ArH), 7.77-7.83ppm (2H, m, 2ArH); 31P NMR(121MHz, CDCl3) -23.1ppm
Lead Reference
R.A. Baber, M.L. Clarke, K. Heslop, A. Marr, A.G. Orpen, P.G. Pringle, A.M. Ward and D.A. Zambrano-Williams, Dalton Trans., 2005, 1079.
Other References
M. Epstein and S.A. Buckler, J. Am. Chem. Soc., 1961, 83, 3279.
M.L. Clarke and G.J. Roff, Chem Eur. J., 2006, 12, 7978.