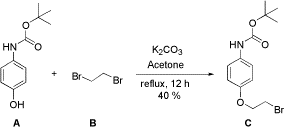
Mono-Alkylation of a Phenol with 1,2-Dibromoethane via Williamson Ether Synthesis
SyntheticPage 253
DOI:
Submitted: January 10, 2007, published: January 11, 2007
Authors
Sandeep Sundriyal (sandeep.niper@gmail.com)
Chemicals
tert-butyl-4-hydroxyphenylcarbamate (lead reference)
1, 2-dibromoethane (Aldrich)
Acetone (Laboratory reagent)
Potassium carbonate anhydrous
1, 2-dibromoethane (Aldrich)
Acetone (Laboratory reagent)
Potassium carbonate anhydrous
Procedure
To a soution of tert-butyl-4-hydroxyphenylcarbamate (A, 0.92g, 4.4mmol) in acetone (10 mL) was added potassium carbonate anhydrous (1.82g, 13.2mmol). The reaction mixture was stirred for 10 min at room temperature after which 1,2-dibromoethane (B, 2.48g, 1.14mL, 13.2mmol) was added. The reaction mixture was then heated at reflux for 12h after which acetone was evaporated and water was added to the residue. The aqueous layer was then extracted with ethyl acetate thrice (10 mL each). The organic washings were combined, washed with brine and dried over anhydrous sodium sulphate. Finally, the organic layer was dried in vacuo to afford solid residue which was purified on silica gel (100g, 60-120 mesh size) eluting with ethylacetate-hexane solvent system. The product was eluted with 2% ethylacetate as a white powder (C, 0.60g, 40%) while the unreacted starting material (A, 0.49g) was obtained with 6% ethylacetate.
Author Comments
The product (C) was required to synthesize a variety of aromatic amines. The former was later derivatized with various O- or N-nucleophiles to achieve this goal. Conversion rate of the given reaction was slow and further increase in reaction time did not improve the conversion. The reaction gives the monoalkylated product selectively and dialkylated product was not observed under the given conditions. All the unreacted starting material (A) can be recovered completely after column chromatography. Both product (C) and starting material (A) have quite different Rf values which ensures their easy separation. The reaction was carried out three times at different scales with almost same isolated yield. The starting material was synthesized by chemoselective tert-butoxycarbonylation of para-amino phenol using method reported in the lead reference.
Data
NMR: 1H NMR 300 MHz (CDCl3): 7.41 (2H, d, J = 6.6Hz), 6.99 (2H, d, J = 6.6Hz), 6.49 (1H, s, D2O exchangable), 4.39 (2H, t, J = 6.3Hz), 3.75 (2H, t, J = 6.3Hz), 1.65 (9H, s)
TLC: Aluminium coated plates (Merck, silica 60 F254); solvent system 10% ethylacetate in hexane; product (C) Rf value = 0.6; starting material Rf value = 0.0.
Lead Reference
Org. Biomol. Chem. 2006, 4(14), 2769-71
Keywords
Comments
I think you might get improve the yield by switching from acetone to DMF. -VH
By Vedran Hasimbegovic on June 30, 2007
I do agree with the comments by Vedran Hasimbegovic but DMF used should be dry and work should be done in Dry Nitrogen atmosphere.The temperature may be in the range of 100 - 120 0C.
By Matloob Ahmad on October 21, 2007
Try cesium carbonate instead of potassium carbonate and DMF instead of acetone to improve the yield. Reaction temperature should be between 70-80 oC. (Cesium carbonate is more soluble than potassium carbonate).
By Prabhu Mohapatra on April 3, 2008