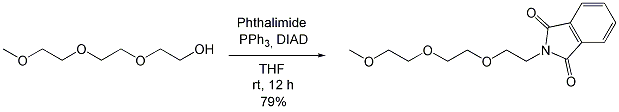
Conversion of Polyethylene Glycol (PEG) to N-(3,6,9-Trioxadecyl)phthalimide (Mitsunobu Reaction)
SyntheticPage 250
DOI:
Submitted: August 18, 2006, published: August 22, 2006
Authors
Vladimir Cmiljanovic (V.Cmiljanovic@stud.unibas.ch)
A contribution from
Chemicals
Phthalimide (Sigma-Aldrich), Triphenylphosphine (PPh3) (Sigma-Aldrich), absolute Tetrahydrofuran (THF) (Sigma), Triethylene glycol monomethyl ether (Fluka), Diisopropyl azodicarboxylate (DIAD) (Fluka), Petroleum ether (Fluka), Ethanol (Fluka), Dichlormethane (Fluka)
Procedure
Phthalimide (3.60 g, 24.5 mmol, 1.2 eq.) and PPh3 (6.42 g, 24.5 mmol, 1.2 eq) were placed under argon in a 2-neck-flask, and dissolved in absolute THF (100 ml). The solution was treated with triethylene glycol monomethyl ether (3.2 ml, 20.4 mmol, 1.0 eq.). After 15 min, DIAD (4.73 ml, 24.5 mmol, 1.2 eq.) was added dropwise at rt., resulting in a slight warming of the solution. After 12 h at rt., TLC showed that the reaction was complete (Hexan-EtOAc/1:1, product Rf 0.31, start material Rf 0.1). The reaction was quenched by addition of EtOH (40 ml). The solvent was evaporated in vacuo and the residue dried at 1 mbar and 40°C for 1 h. The residue was treated with 20 ml petroleum ether (PE)-EtOAc (1 : 1) and stirred at 40°C for 1 h. The white residue was filtered off and washed with 10 ml PE-EtOAc (1 : 1). The filtrate was evaporated to dryness, dried at high vacuum, dissolved in 1 ml dichlormethane and chromatographed on silica gel (10-50% EtOAc in hexane). In this way, the title compound was synthesised as a colorless oil (4.73 g, 16.1 mmol, 79 %).
Author Comments
This reaction was repeated several times and can be non-problematic carried out on a multigramm scale.
PEGylation: Attaching polyethylene glycol, or PEGylation as it is commonly known, can improve the water solubility of diverse small organic molecules, the stability of a target protein, protect it from proteolytic digestion, improve protein solubility and reduce the tendency of the modified protein to aggregate. In this case the -OH group of the PEG will be converted in -NH2 group in a two-step synthesis (for the second step see the synthesis of 3,6,9-Trioxadecylamine )
Data
TLC (Hexan-EtOAc/1:1): Rf = 0.31.
1H NMR (CDCl3, 400 MHz): 7.80 (dd, J = 3.0 Hz, J = 5.5 Hz, 2H), 7.68 (dd, J = 3.0 Hz, J = 5.5 Hz, 2 H), 3.87 (t, J = 5.8 Hz, 2 H), 3.71 (t, J = 5.8 Hz, 2 H), 3.64 – 3.54 (m, 6 H), 3.45 – 3.42 (m, 2 H), 3.30 (s, 3 H).
13C-NMR (CDCl3, 125 MHz): 168.12, 133.79, 132.01, 123.09, 71.75, 70.44, 70.41, 69.99, 67.79, 58.87, 37.15.
Lead Reference
Synthesis (6), 816-824, 2002.
Keywords
Comments
Good Idea
By youth huang on June 20, 2007